 W
WAlliin is a sulfoxide that is a natural constituent of fresh garlic. It is a derivative of the amino acid cysteine. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic.
 W
WS-Allylcysteine (SAC) is an organosulfur compound that is a constituent of fresh garlic. It is a derivative of the amino acid cysteine in which an allyl group replaces the proton on sulfur. A number of related compounds are found in garlic, including the disulfide S-"allylmercaptocysteine" (SAMC) and γ-glutamyl-S-cysteine" (GSAC).
 W
WS-Aminoethyl-l-cysteine, also known as thialysine, is a toxic analog of the amino acid lysine in which the second carbon of the amino acid's R-group has been replaced with a sulfur atom.
 W
WCystathionine is an intermediate in the synthesis of cysteine.
 W
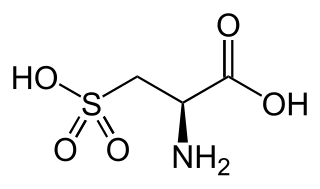
WCysteic acid also known as 3-sulfo-l-alanine is the organic compound with the formula HO3SCH2CH(NH2)CO2H. It is often referred to as cysteate, which near neutral pH takes the form −O3SCH2CH(NH3+)CO2−.
 W
WCysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula HO2CCH(NH2)CH2SH. The thiol side chain in cysteine often participates in enzymatic reactions, as a nucleophile. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. When used as a food additive, it has the E number E920. It is encoded by the codons UGU and UGC.
 W
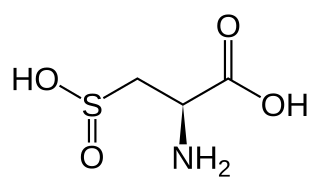
WCysteine sulfinic acid, also known as 3-sulfino-L-alanine, is an amino acid containing a sulfinic acid functional group. It is a white solid that is soluble in water. It is an intermediate in cysteine metabolism. It is not a coded amino acid, but is produced post-translationally.
 W
WCysteinyldopa is a catecholamine. Excessive cysteinyldopa in plasma and urine has been linked to malignant melanoma. Cysteinyldopa is found in large amounts in the plasma and urine of patients with malignant melanoma. It is therefore used in the diagnosis of melanoma and for the detection of postoperative metastases. Cysteinyldopa is believed to be formed by the rapid enzymatic hydrolysis of 5-S-glutathionedopa found in melanin-producing cells.
 W
WCystine is the oxidized dimer form of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is slightly soluble in water. It serves two biological functions: a site of redox reactions and a mechanical linkage that allows proteins to retain their three-dimensional structure.
 W
WDjenkolic acid is a sulfur-containing non-protein amino acid naturally found in djenkol beans of the South-East Asian legumes jengkol. Its chemical structure is similar to cystine but contains a methylene unit in between the two sulfur atoms.
 W
WEthionine is a non-proteinogenic amino acid structurally related to methionine, with an ethyl group in place of the methyl group.
 W
WFelinine, also known as (R)-2-amino-3-(4-hydroxy-2-methylbutan-2-ylthio)propanoic acid, is a chemical compound and amino acid found in cat urine and a precursor via microbial lyase of the putative cat pheromone and thiol called 3-mercapto-3-methylbutan-1-ol (MMB). Felinine is excreted by selected Felidae species including bobcats, Chinese desert cats, the kodkod, and domestic cats. Felinine synthesis occurs in the liver through a condensation reaction of glutathione and isopentenyl pyrophosphate to form 3-mercaptobutanolglutathionine (3-MBG). In the kidney 3-MBG is hydrolysed and felinine partly acetylated. Cauxin assists in the hydrolysis of the dipeptide (felinylglycine) to increase the concentration of urinary felinine. Urine of domestic cats may contain a series of felinine-containing compounds including free felinine, acetylfelinine, felinylglycine and 3-MBG.
 W
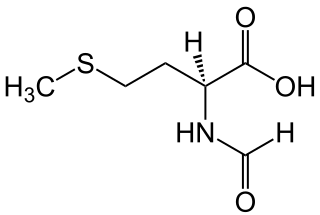
WN-Formylmethionine is a derivative of the amino acid methionine in which a formyl group has been added to the amino group. It is specifically used for initiation of protein synthesis from bacterial and organellar genes, and may be removed post-translationally.
 W
WHawkinsin (also known as 2-cystenyl-1,4-dihydroxycyclohexenylacetate) is an amino acid, which is formed after detoxification of a reactive tyrosine metabolite (quinol acetate) by glutathione. Hawkinsin is ninhydrin positive (a common test to detect amino acids and proteins with a free -NH2 group).
 W
WHomocysteine is a non-proteinogenic α-amino acid. It is a homologue of the amino acid cysteine, differing by an additional methylene bridge (-CH2-). It is biosynthesized from methionine by the removal of its terminal Cε methyl group. In the body, homocysteine can be recycled into methionine or converted into cysteine with the aid of certain B-vitamins.
 W
WLanthionine is a nonproteinogenic amino acid with the chemical formula (HOOC-CH(NH2)-CH2-S-CH2-CH(NH2)-COOH). It is typically formed by a cysteine residue and a dehydrated serine residue. Despite its name, lanthionine does not contain the element lanthanum.
 W
WLanthionine ketimine is a naturally occurring sulfur amino acid metabolite found in the mammalian brain and central nervous system (CNS).
 W
WMethionine is an essential amino acid in humans. As the substrate for other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is encoded by the codon AUG.
 W
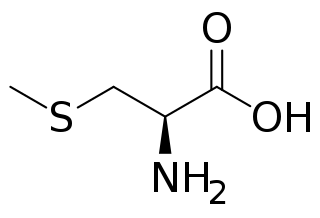
WS-Methylcysteine is the amino acid with the nominal formula CH3SCH2CH(NH2)CO2H. It is the S-methylated derivative of cysteine. This amino acid occurs widely in plants, including many edible vegetables.
 W
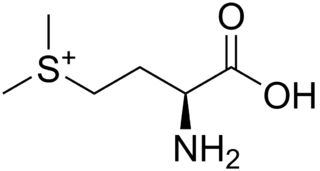
WS-Methylmethionine (SMM) is a derivative of methionine with the chemical formula (CH3)2S+CH2CH2CH(NH3+)CO2−. This cation is a naturally-occurring intermediate in many biosynthetic pathways owing to the sulfonium functional group. It is biosynthesized from L-methionine which is first converted to S-adenosylmethionine. The subsequent conversion, involving replacement of the adenosyl group by a methyl group is catalyzed by the enzyme methionine S-methyltransferase. S-methylmethionine is particularly abundant in plants, being more abundant than methionine.