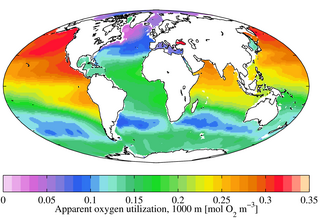
 W
WIn freshwater or marine systems apparent oxygen utilization (AOU) is the difference between oxygen gas solubility and the measured oxygen concentration in water with the same physical and chemical properties. Such differences typically occur when biological activity acts to change the ambient concentration of oxygen. For example, primary production liberates oxygen and increases its concentration, while respiration consumes it and decreases its concentration.
 W
WIn ecology and Earth science, a biogeochemical cycle or substance turnover or cycling of substances is a pathway by which a chemical substance moves through biotic (biosphere) and abiotic compartments of Earth. There are biogeochemical cycles for the chemical elements calcium, carbon, hydrogen, mercury, nitrogen, oxygen, phosphorus, selenium, iron and sulfur; molecular cycles for water and silica; macroscopic cycles such as the rock cycle; as well as human-induced cycles for synthetic compounds such as polychlorinated biphenyl (PCB). In some cycles there are reservoirs where a substance remains for a long period of time.
 W
WA Bjerrum plot (named after Niels Bjerrum) is a graph of the concentrations of the different species of a polyprotic acid in a solution, as a function of pH, when the solution is at equilibrium. Due to the many orders of magnitude spanned by the concentrations, they are commonly plotted on a logarithmic scale. Sometimes the ratios of the concentrations are plotted rather than the actual concentrations. Occasionally H+ and OH− are also plotted.
 W
WThe carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. Carbon is the main component of biological compounds as well as a major component of many minerals such as limestone. Along with the nitrogen cycle and the water cycle, the carbon cycle comprises a sequence of events that are key to make Earth capable of sustaining life. It describes the movement of carbon as it is recycled and reused throughout the biosphere, as well as long-term processes of carbon sequestration to and release from carbon sinks.
 W
WThe carbonate–silicate geochemical cycle, also known as the inorganic carbon cycle, describes the long-term transformation of silicate rocks to carbonate rocks by weathering and sedimentation, and the transformation of carbonate rocks back into silicate rocks by metamorphism and volcanism. Carbon dioxide is removed from the atmosphere during burial of weathered minerals and returned to the atmosphere through volcanism. On million-year time scales, the carbonate-silicate cycle is a key factor in controlling Earth's climate because it regulates carbon dioxide levels and therefore global temperature.
 W
WChemical cycling describes systems of repeated circulation of chemicals between other compounds, states and materials, and back to their original state, that occurs in space, and on many objects in space including the Earth. Active chemical cycling is known to occur in stars, many planets and natural satellites.
 W
WWetland chemistry is largely affected by dredging, which can be done for a variety of purposes. Wetlands are areas within floodplains with both terrestrial and aquatic characteristics, including marshes, swamps, bogs, and others. It has been estimated that they occupy around 2.8x106 km2, about 2.2% of the earth’s surface, but other estimates are even higher. It has also been estimated to have a worth of $14.9 trillion and are responsible for 75% of commercial and 90% of recreational harvest of fish and shellfish in the United States. Wetlands also hold an important role in water purification, storm protection, industry, travel, research, education, and tourism. Being heavily used and traveled through, dredging is common and leads to continuation of long-term damage of the ecosystem and land loss, and ultimately a loss in industry, homes, and protection.
 W
WClimate change mitigation consists of actions to limit the magnitude or rate of global warming and its related effects. This generally involves reductions in human emissions of greenhouse gases (GHGs).
 W
WIn petrology, the mineral clinopyroxene is used for temperature and pressure calculations of the magma that produced igneous rock containing this mineral. Clinopyroxene thermobarometry is one of several geothermobarometers. Two things makes this method especially useful: first, clinopyroxene is a common phenocryst in igneous rocks and easy to identify; second, the crystallization of the jadeite component of clinopyroxene implies a growth in molar volume being thus a good indicator of pressure.
 W
WCompatibility is a term used by geochemists to describe how elements partition themselves in the solid and melt within Earth's mantle. In geochemistry, compatibility is a measure of how readily a particular trace element substitutes for a major element within a mineral.
 W
WThe composition of Mars covers the branch of the geology of Mars that describes the make-up of the planet Mars.
 W
WIn oceanic biogeochemistry, the continental shelf pump is proposed to operate in the shallow waters of the continental shelves, acting as a mechanism to transport carbon from surface waters to the interior of the adjacent deep ocean.
 W
WDallol is a unique, terrestrial hydrothermal system in Ethiopia. It is known for its unearthly colors and mineral patterns, and the very acidic fluids that discharge from its hydrothermal springs.
 W
WThe deep carbon cycle is the movement of carbon through the Earth's mantle and core. It forms part of the carbon cycle and is intimately connected to the movement of carbon in the Earth's surface and atmosphere. By returning carbon to the deep Earth, it plays a critical role in maintaining the terrestrial conditions necessary for life to exist. Without it, carbon would accumulate in the atmosphere, reaching extremely high concentrations over long periods of time.
 W
WIn geochemistry, paleoclimatology, and paleoceanography δ13C is an isotopic signature, a measure of the ratio of stable isotopes 13C : 12C, reported in parts per thousand. The measure is also widely used in archaeology for the reconstruction of past diets, particularly to see if marine foods or certain types of plants were consumed
 W
WThe δ34S value is a standardized method for reporting measurements of the ratio of two stable isotopes of sulfur, 34S:32S, in a sample against the equivalent ratio in a known reference standard. Presently, the most commonly used standard is Vienna-Canyon Diablo Troilite (VCDT). Results are reported as variations from the standard ratio in parts per thousand, per mil or per mille, using the ‰ symbol. Heavy and light sulfur isotopes fractionate at different rates and the resulting δ34S values, recorded in marine sulfate or sedimentary sulfides, have been studied and interpreted as records of the changing sulfur cycle throughout the earth's history.
 W
WDissolved load is the portion of a stream's total sediment load that is carried in solution, especially ions from chemical weathering. It is a major contributor to the total amount of material removed from a river's drainage basin, along with suspended load and bed load. The amount of material carried as dissolved load is typically much smaller than the suspended load, though this is not always the case, particularly when the available river flow is mostly harnessed for purposes such as irrigation or industrial uses. Dissolved load comprises a significant portion of the total material flux out of a landscape, and its composition is important in regulating the chemistry and biology of the stream water.
 W
WThe Europium anomaly is the phenomenon whereby the europium (Eu) concentration in a mineral is either enriched or depleted relative to some standard, commonly a chondrite or mid-ocean ridge basalt (MORB). In geochemistry a europium anomaly is said to be "positive" if the Eu concentration in the mineral is enriched relative to the other rare-earth elements (REEs), and is said to be "negative" if Eu is depleted relative to the other REEs.
 W
WA fluid inclusion is a microscopic bubble of liquid and gas that is trapped within a crystal. As minerals often form from a liquid or aqueous medium, tiny blebs of that liquid can become trapped within the crystal, or along healed crystal fractures. These small inclusions range in size from 0.01 to 1 mm and are usually only visible in detail by microscopic study.
 W
WFulgurites are natural tubes, clumps, or masses of sintered, vitrified, and/or fused soil, sand, rock, organic debris and other sediments that sometimes form when lightning discharges into ground. Fulgurites are classified as a variety of the mineraloid lechatelierite. When ordinary negative polarity cloud-ground lightning discharges into a grounding substrate, greater than 100 million volts of potential difference may be bridged. Such current may propagate into silica-rich quartzose sand, mixed soil, clay, or other sediments, rapidly vaporizing and melting resistant materials within such a common dissipation regime. This results in the formation of generally hollow and/or vesicular, branching assemblages of glassy tubes, crusts, and clumped masses. Fulgurites have no fixed composition because their chemical composition is determined by the physical and chemical properties of whatever material is being struck by lightning.
 W
WGeopolymer cement is a binding system that hardens at room temperature.
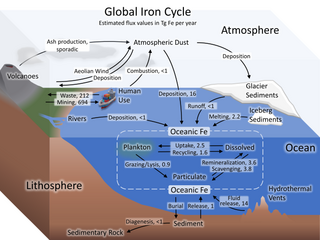 W
WThe iron cycle (Fe) is the biogeochemical cycle of iron through the atmosphere, hydrosphere, biosphere and lithosphere. While Fe is highly abundant in the Earth's crust, it is less common in oxygenated surface waters. Iron is a key micronutrient in primary productivity, and a limiting nutrient in the Southern ocean, eastern equatorial Pacific, and the subarctic Pacific referred to as High-Nutrient, Low-Chlorophyll (HNLC) regions of the ocean.
 W
WIsotope-ratio mass spectrometry (IRMS) is a specialization of mass spectrometry, in which mass spectrometric methods are used to measure the relative abundance of isotopes in a given sample.
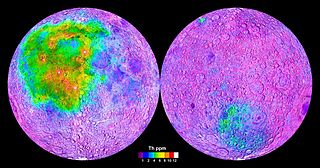 W
WKREEP, an acronym built from the letters K, REE and P, is a geochemical component of some lunar impact breccia and basaltic rocks. Its most significant feature is somewhat enhanced concentration of a majority of so-called "incompatible" elements and the heat-producing elements, namely radioactive uranium, thorium, and potassium.
 W
WThe Moon is composed of two major geologic provinces that have a unique origin, composition, and thermal evolution. The Procellarum KREEP Terrane is a large province on the near side of the Moon that has high abundances of KREEP. KREEP, an acronym built from the letters K, REE and P, is a geochemical component of some lunar impact breccia and basaltic rocks. The Felspathic Highlands Terrane, in contrast is composed predominantly of ancient anorthositic materials. A third terrane, the South Pole–Aitken Terrane, may simply represent deep crustal materials of the feldspathic highlands terrane.
 W
WThe lysocline is the depth in the ocean dependent upon the calcite compensation depth (CCD), usually around 3.5 km, below which the rate of dissolution of calcite increases dramatically because of a pressure effect. While the lysocline is the upper bound of this transition zone of calcite saturation, the CCD is the lower bound of this zone.
 W
WMantle oxidation state applies the concept of oxidation state in chemistry to the study of the Earth's mantle. The chemical concept of oxidation state mainly refers to the valence state of one element, while mantle oxidation state provides the degree of decreasing of increasing valence states of all polyvalent elements in mantle materials confined in a closed system. The mantle oxidation state is controlled by oxygen fugacity and can be benchmarked by specific groups of redox buffers.
 W
WMarine biogeochemical cycles are biogeochemical cycles that occur within marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. These biogeochemical cycles are the pathways chemical substances and elements move through within the marine environment. In addition, substances and elements can be imported into or exported from the marine environment. These imports and exports can occur as exchanges with the atmosphere above, the ocean floor below, or as runoff from the land.
 W
WIn the deep ocean, marine snow is a continuous shower of mostly organic detritus falling from the upper layers of the water column. It is a significant means of exporting energy from the light-rich photic zone to the aphotic zone below which is referred to as the biological pump. Export production is the amount of organic matter produced in the ocean by primary production that is not recycled (remineralised) before it sinks into the aphotic zone. Because of the role of export production in the ocean's biological pump, it is typically measured in units of carbon .The term was first coined by the explorer William Beebe as he observed it from his bathysphere. As the origin of marine snow lies in activities within the productive photic zone, the prevalence of marine snow changes with seasonal fluctuations in photosynthetic activity and ocean currents. Marine snow can be an important food source for organisms living in the aphotic zone, particularly for organisms which live very deep in the water column.
 W
WA melt inclusion is a small parcel or "blobs" of melt(s) that is entrapped by crystals growing in magma and eventually forming igneous rocks. In many respects it is analogous to a fluid inclusion within magmatic hydrothermal systems. Melt inclusions tend to be microscopic in size and can be analyzed for volatile contents that are used to interpret trapping pressures of the melt at depth.
 W
WA micrometeorite is a micrometeoroid that has survived entry through the Earth's atmosphere. The IAU officially defines meteorites as 30 micrometers to 1 meter; micrometeorites are the small end of the range (~submillimeter). Usually found on Earth's surface, micrometeorites differ from meteorites in that they are smaller in size, more abundant, and different in composition. They are a subset of cosmic dust, which also includes the smaller interplanetary dust particles (IDPs).
 W
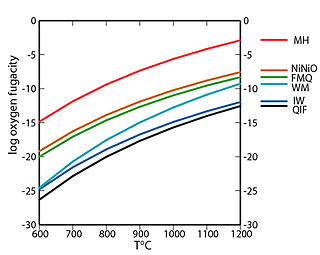
WIn geology, a redox buffer is an assemblage of minerals or compounds that constrains oxygen fugacity as a function of temperature. Knowledge of the redox conditions (or equivalently, oxygen fugacities) at which a rock forms and evolves can be important for interpreting the rock history. Iron, sulfur, and manganese are three of the relatively abundant elements in the Earth's crust that occur in more than one oxidation state. For instance, iron, the fourth most abundant element in the crust, exists as native iron, ferrous iron (Fe2+), and ferric iron (Fe3+). The redox state of a rock affects the relative proportions of the oxidation states of these elements and hence may determine both the minerals present and their compositions. If a rock contains pure minerals that constitute a redox buffer, then the oxygen fugacity of equilibration is defined by one of the curves in the accompanying fugacity-temperature diagram.
 W
WOcean acidification is the ongoing decrease in the pH of the Earth's oceans, caused by the uptake of carbon dioxide (CO2) from the atmosphere. Seawater is slightly basic (meaning pH > 7), and ocean acidification involves a shift towards pH-neutral conditions rather than a transition to acidic conditions (pH < 7). An estimated 30–40% of the carbon dioxide from human activity released into the atmosphere dissolves into oceans, rivers and lakes. Some of it reacts with the water to form carbonic acid. Some of the resulting carbonic acid molecules dissociate into a bicarbonate ion and a hydrogen ion, thus increasing ocean acidity (H+ ion concentration). Between 1751 and 1996, surface ocean pH is estimated to have decreased from approximately 8.25 to 8.14, representing an increase of almost 30% in H+ ion concentration in the world's oceans. Earth System Models project that, by around 2008, ocean acidity exceeded historical analogues and, in combination with other ocean biogeochemical changes, could undermine the functioning of marine ecosystems and disrupt the provision of many goods and services associated with the ocean beginning as early as 2100.
 W
WOcean acidification threatens the Great Barrier Reef by reducing the viability and strength of coral reefs. The Great Barrier Reef, considered one of the seven natural wonders of the world and a biodiversity hotspot, is located in Australia. Similar to other coral reefs, it is experiencing degradation due to ocean acidification. Ocean acidification results from a rise in atmospheric carbon dioxide, which is taken up by the ocean. This process can increase sea surface temperature, decrease aragonite, and lower the pH of the ocean.
 W
WThe oceanic carbon cycle is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle is a result of many interacting forces across multiple time and space scales that circulates carbon around the planet, ensuring that carbon is available globally. The Oceanic carbon cycle is a central process to the global carbon cycle and contains both inorganic carbon and organic carbon. Part of the marine carbon cycle transforms carbon between non-living and living matter.
 W
WVarious theories of ore genesis explain how the various types of mineral deposits form within the Earth's crust. Ore-genesis theories vary depending on the mineral or commodity examined.
 W
WPhotogeochemistry merges photochemistry and geochemistry into the study of light-induced chemical reactions that occur or may occur among natural components of Earth's surface. The first comprehensive review on the subject was published in 2017 by the chemist and soil scientist Timothy A Doane, but the term photogeochemistry appeared a few years earlier as a keyword in studies that described the role of light-induced mineral transformations in shaping the biogeochemistry of Earth; this indeed describes the core of photogeochemical study, although other facets may be admitted into the definition.
 W
WPit water, mine water or mining water is water that collects in a mine and which has to be brought to the surface by water management methods in order to enable the mine to continue working.
 W
WIn geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the interstellar medium from which the solar system was formed, and were formed in, or after, the Big Bang, by nucleosynthesis in stars and supernovae followed by mass ejection, by cosmic ray spallation, and potentially from other processes. They are the stable nuclides plus the long-lived fraction of radionuclides surviving in the primordial solar nebula through planet accretion until the present. Only 286 such nuclides are known.
 W
WIn oceanic biogeochemistry, the solubility pump is a physico-chemical process that transports carbon as dissolved inorganic carbon (DIC) from the ocean's surface to its interior.
 W
WTephrochronology is a geochronological technique that uses discrete layers of tephra—volcanic ash from a single eruption—to create a chronological framework in which paleoenvironmental or archaeological records can be placed. Such an established event provides a "tephra horizon". The premise of the technique is that each volcanic event produces ash with a unique chemical "fingerprint" that allows the deposit to be identified across the area affected by fallout. Thus, once the volcanic event has been independently dated, the tephra horizon will act as time marker.
 W
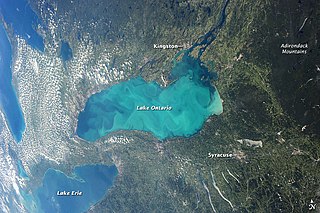
WA whiting event is a phenomenon that occurs when a suspended cloud of fine-grained calcium carbonate precipitates in water bodies, typically during summer months, as a result of photosynthetic microbiological activity or sediment disturbance. The phenomenon gets its name from the white, chalky color it imbues to the water. These events have been shown to occur in temperate waters as well as tropical ones, and they can span for hundreds of meters. They can also occur in both marine and freshwater environments. The origin of whiting events is debated among the scientific community, and it is unclear if there is a single, specific cause. Generally, they are thought to result from either bottom sediment re-suspension or by increased activity of certain microscopic life such as phytoplankton. Because whiting events affect aquatic chemistry, physical properties, and carbon cycling, studying the mechanisms behind them holds scientific relevance in various ways.