 W
WThe copper coulometer is a one application for the copper-copper(II) sulfate electrode. Such a coulometer consists of two identical copper electrodes immersed in slightly acidic pH-buffered solution of copper(II) sulfate. Passing of current through the element leads to the anodic dissolution of the metal on anode and simultaneous deposition of copper ions on the cathode. These reactions have 100% efficiency over a wide range of current density.
 W
WThe dropping mercury electrode (DME) is a working electrode made of mercury and used in polarography. Experiments run with mercury electrodes are referred to as forms of polarography even if the experiments are identical or very similar to a corresponding voltammetry experiment which uses solid working electrodes. Like other working electrodes these electrodes are used in electrochemical studies using three electrode systems when investigating reaction mechanisms related to redox chemistry among other chemical phenomena.
 W
WThe hanging mercury drop electrode is a working electrode variation on the dropping mercury electrode (DME). Experiments run with dropping mercury electrodes are referred to as forms of polarography. If the experiments are performed at an electrode with a constant surface it is referred as voltammetry.
 W
WA mercury coulometer is an electroanalytical chemistry device using mercury to determine the amount of matter transformed during the following reaction:
 W
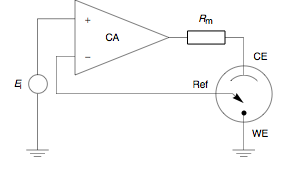
WA potentiostat is the electronic hardware required to control a three electrode cell and run most electroanalytical experiments. A Bipotentiostat and polypotentiostat are potentiostats capable of controlling two working electrodes and more than two working electrodes, respectively.
 W
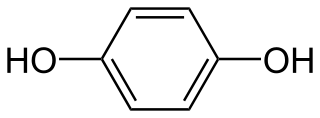
WThe quinhydrone electrode may be used to measure the hydrogen ion concentration (pH) of a solution containing an acidic substance.
 W
WA voltameter or coulometer is a scientific instrument used for measuring electric charge through electrolytic action. The SI unit of electric charge is the coulomb.