 W
WAtomic electron transition is a change of an electron from one energy level to another within an atom or artificial atom. It appears discontinuous as the electron "jumps" from one quantized energy level to another, typically in a few nanoseconds or less. It is also known as an electronic (de-)excitation or atomic transition or quantum jump.
 W
WIn atomic theory and quantum mechanics, an atomic orbital is a mathematical function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term atomic orbital may also refer to the physical region or space where the electron can be calculated to be present, as predicted by the particular mathematical form of the orbital.
 W
WThe aufbau principle, from the German Aufbauprinzip, also called the aufbau rule, states that in the ground state of an atom or ion, electrons fill subshells of the lowest available energy, then they fill subshells of higher energy. For example, the 1s subshell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable electron configuration possible. An example is the configuration 1s2 2s2 2p6 3s2 3p3 for the phosphorus atom, meaning that the 1s subshell has 2 electrons, and so on.
 W
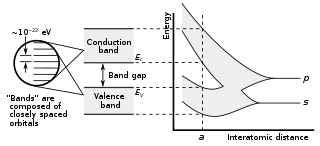
WIn solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote a valence electron bound to an atom to become a conduction electron, which is free to move within the crystal lattice and serve as a charge carrier to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move in the solid; however, if some electrons transfer from the valence to the conduction band, then current can flow. Therefore, the band gap is a major factor determining the electrical conductivity of a solid. Substances with large band gaps are generally insulators, those with smaller band gaps are semiconductors, while conductors either have very small band gaps or none, because the valence and conduction bands overlap.
 W
WIn chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond. Term delocalization is general and can have slightly different meanings in different fields. In organic chemistry, this refers to resonance in conjugated systems and aromatic compounds. In solid-state physics, this refers to free electrons that facilitate electrical conduction. In quantum chemistry, this refers to molecular orbital electrons that have extended over several adjacent atoms.
 W
WIn atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, meaning that the 1s, 2s and 2p subshells are occupied by 2, 2 and 6 electrons respectively.
 W
WApplying line voltage across a pickled cucumber causes it to glow. A moist pickle contains salt as a result of the pickling process, which allows it to conduct electricity. Sodium ions within the pickle emit light as a result of atomic electron transitions, although it is not clear why the luminescence occurs at one end of the pickle.
 W
WSecondary electrons are electrons generated as ionization products. They are called 'secondary' because they are generated by other radiation. This radiation can be in the form of ions, electrons, or photons with sufficiently high energy, i.e. exceeding the ionization potential. Photoelectrons can be considered an example of secondary electrons where the primary radiation are photons; in some discussions photoelectrons with higher energy (>50 eV) are still considered "primary" while the electrons freed by the photoelectrons are "secondary".
 W
WIn chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair.