 W
WThis page is a list of notable battery types grouped by types of battery.
 W
WA battery pack is a set of any number of (preferably) identical batteries or individual battery cells. They may be configured in a series, parallel or a mixture of both to deliver the desired voltage, capacity, or power density. The term battery pack is often used in reference to cordless tools, radio-controlled hobby toys, and battery electric vehicles.
 W
WThe Bunsen cell is a zinc-carbon primary cell composed of a zinc anode in dilute sulfuric acid separated by a porous pot from a carbon cathode in nitric or chromic acid.
 W
WThe Chromic acid cell was a type of primary cell which used chromic acid as a depolarizer. The chromic acid was usually made by acidifying a solution of potassium dichromate. The old name for potassium dichromate was potassium bichromate and the cell was often called a Bichromate cell. This type of cell is now only of historical interest.
 W
WThe Clark cell, invented by English engineer Josiah Latimer Clark in 1873, is a wet-chemical cell that produces a highly stable voltage. In 1893, the output of the Clark cell at 15 °C was defined by the International Electrical Congress as 1.434 volts, and this definition became law in the United States in 1894. This definition was later supplanted by one based on the Weston cell.
 W
WThe Daniell cell is a type of electrochemical cell invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consists of a copper pot filled with a copper (II) sulfate solution, in which is immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode. He was searching for a way to eliminate the hydrogen bubble problem found in the voltaic pile, and his solution was to use a second electrolyte to consume the hydrogen produced by the first. Zinc sulfate may be substituted for the sulfuric acid. The Daniell cell was a great improvement over the existing technology used in the early days of battery development. A later variant of the Daniell cell called the gravity cell or crowfoot cell was invented in the 1860s by a Frenchman named Callaud and became a popular choice for electrical telegraphy.
 W
WA deep-cycle battery is a battery designed to be regularly deeply discharged using most of its capacity. The term is traditionally mainly used for lead–acid batteries in the same form factor as automotive batteries; and contrasted with starter or 'cranking' automotive batteries designed to deliver only a small part of their capacity in a short, high-current burst for cranking the engine.
 W
WThe Edison–Lalande cell was a type of alkaline primary battery developed by Thomas Edison from an earlier design by Felix Lalande and Georges Chaperon. It consisted of plates of copper oxide and zinc in a solution of potassium hydroxide. The cell voltage was low but the internal resistance was also low so these cells were capable of delivering large currents.
 W
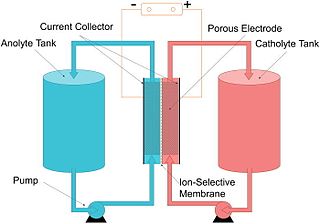
WA flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion exchange occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts.
 W
WA frog battery is an electrochemical battery consisting of a number of dead frogs, which form the cells of the battery connected in a series arrangement. It is a kind of biobattery. It was used in early scientific investigations of electricity and academic demonstrations.
 W
WThe Leclanché cell is a battery invented and patented by the French scientist Georges Leclanché in 1866. The battery contained a conducting solution (electrolyte) of ammonium chloride, a cathode of carbon, a depolarizer of manganese dioxide (oxidizer), and an anode of zinc (reductant). The chemistry of this cell was later successfully adapted to manufacture a dry cell.
 W
WMolten-salt batteries are a class of battery that uses molten salts as an electrolyte and offers both a high energy density and a high power density. Traditional non-rechargeable thermal batteries can be stored in their solid state at room temperature for long periods of time before being activated by heating. Rechargeable liquid-metal batteries are used for industrial power backup, special electric vehicles and for grid energy storage, to balance out intermittent renewable power sources such as solar panels and wind turbines.
 W
WNanobatteries are fabricated batteries employing technology at the nanoscale, particles that measure less than 100 nanometers or 10−7 meters. These batteries may be nano in size or may use nanotechnology in a macro scale battery. Nanoscale batteries can be combined together to function as a macrobattery such as within a nanopore battery.
 W
WThe penny battery is a voltaic pile which uses various coinage as the metal disks (pennies) of a traditional voltaic pile. The coins are stacked with pieces of electrolyte soaked paper in between. The penny battery experiment is common during electrochemistry units in an educational setting.
 W
WSolid dispersion redox flow battery is one specific type of redox flow battery using dispersed solid active materials as the energy storage media. The solid suspensions are stored in energy storage tanks and pumped through electrochemical cells while charging or discharging. In comparison with a conventional redox flow battery where active species are dissolved in aqueous or organic electrolyte, the active materials in a solid dispersion redox flow battery maintain the solid form and are suspended in the electrolyte. Further development expanded the applicable active materials. The solid active materials, especially with active materials from lithium-ion battery, can help the suspensions achieve much higher energy densities than conventional redox flow batteries. This concept is similar to semi-solid flow batteries in which slurries of active materials accompanied by conductive carbon additives to facilitate electrons conducting are stored in energy storage tanks and pumped through the electrochemical reaction cells. Based upon this technique, an analytical method was developed to measure the electrochemical performance of lithium-ion battery active materials, named Dispersed Particle Resistance (DPR).
 W
WA solid-state electrolyte (SSE) is a solid ionic conductor electrolyte and it is the characteristic component of the solid-state battery. It is useful for applications in electrical energy storage (EES) in substitution of the liquid electrolytes found in particular in lithium-ion battery. The main advantages are the increased safety, no issues of leakages of toxic organic liquids, low flammability, non-volatility, mechanical and thermal stability, easy processability, low self-discharge, higher achievable power density and cyclability. This makes it possible, for example, the use of a lithium metal anode in a practical device, without the intrinsic limitations of a liquid electrolyte. The utilization of a high capacity anode and low reduction potential, like lithium with a specific capacity of 3860 mAh g−1 and a reduction potential of -3.04 V vs SHE, in substitution of the traditional low capacity graphite, which exhibits a theoretical capacity of 372 mAh g−1 in its fully lithiated state of LiC6, is the first step in the realization of a lighter, thinner and cheaper rechargeable battery. Moreover this allows the reach of gravimetric and volumetric energy densities, high enough to achieve 500 miles per single charge in an electric vehicle. Despite the promising advantages there are still some limitations that are hindering the transition of SSEs from academic research to large-scale production, however many car OEMs (Toyota, BMW, Honda, Hyundai) expect to integrate these systems into viable devices and to commercialize solid-state battery-based electric vehicles by 2025.
 W
WThe trough battery was a variant of Alessandro Volta's Voltaic Pile and was invented by William Cruickshank c. 1800.
 W
WThe vanadium redox battery (VRB), also known as the vanadium flow battery (VFB) or vanadium redox flow battery (VRFB), is a type of rechargeable flow battery that employs vanadium ions in different oxidation states to store chemical potential energy. The vanadium redox battery exploits the ability of vanadium to exist in solution in four different oxidation states, and uses this property to make a battery that has just one electroactive element instead of two. For several reasons, including their relative bulkiness, most vanadium batteries are currently used for grid energy storage, i.e., attached to power plants or electrical grids.
 W
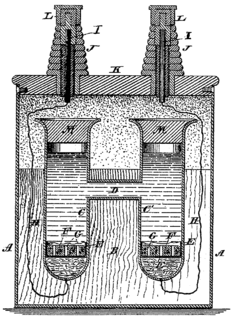
WThe voltaic pile was the first electrical battery that could continuously provide an electric current to a circuit. It was invented by Italian physicist Alessandro Volta, who published his experiments in 1799. The voltaic pile then enabled a rapid series of other discoveries including the electrical decomposition (electrolysis) of water into oxygen and hydrogen by William Nicholson and Anthony Carlisle (1800) and the discovery or isolation of the chemical elements sodium (1807), potassium (1807), calcium (1808), boron (1808), barium (1808), strontium (1808), and magnesium (1808) by Humphry Davy.
 W
WThe Weston cell is a wet-chemical cell that produces a highly stable voltage suitable as a laboratory standard for calibration of voltmeters. Invented by Edward Weston in 1893, it was adopted as the International Standard for EMF from 1911 until superseded by the Josephson voltage standard in 1990.
 W
WThe Zamboni pile is an early electric battery, invented by Giuseppe Zamboni in 1812.
 W
WZinc–air batteries (non-rechargeable), and zinc–air fuel cells are metal–air batteries powered by oxidizing zinc with oxygen from the air. These batteries have high energy densities and are relatively inexpensive to produce. Sizes range from very small button cells for hearing aids, larger batteries used in film cameras that previously used mercury batteries, to very large batteries used for electric vehicle propulsion and grid-scale energy storage.
 W
WA zinc–carbon battery is a dry cell primary battery that provides direct electric current from the electrochemical reaction between zinc and manganese dioxide. It produces a voltage of about 1.5 volts between the zinc anode, which is typically realized as a container for the battery, and a carbon rod of positive polarity, the cathode, that collects the current from the manganese dioxide electrode, giving the cell its name.
 W
WZinc–cerium batteries are a type of redox flow battery first developed by Plurion Inc. (UK) during the 2000s. In this rechargeable battery, both negative zinc and positive cerium electrolytes are circulated though an electrochemical flow reactor during the operation and stored in two separated reservoirs. Negative and positive electrolyte compartments in the electrochemical reactor are separated by a cation-exchange membrane, usually Nafion (DuPont). The Ce(III)/Ce(IV) and Zn(II)/Zn redox reactions take place at the positive and negative electrodes, respectively. Since zinc is electroplated during charge at the negative electrode this system is classified as a hybrid flow battery. Unlike in zinc–bromine and zinc–chlorine redox flow batteries, no condensation device is needed to dissolve halogen gases. The reagents used in the zinc-cerium system are considerably less expensive than those used in the vanadium flow battery.