 W
WNuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI).
 W
WAn aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons of the aromatic ring. This is a direct consequence of Ampère's law; since the electrons involved are free to circulate, rather than being localized in bonds as they would be in most non-aromatic molecules, they respond much more strongly to the magnetic field.
 W
WFelix Bloch was a Swiss-American physicist and Nobel physics laureate who worked mainly in the U.S. He and Edward Mills Purcell were awarded the 1952 Nobel Prize for Physics for "their development of new ways and methods for nuclear magnetic precision measurements." In 1954–1955, he served for one year as the first Director-General of CERN. Felix Bloch made fundamental theoretical contributions to the understanding of electron behavior in crystal lattices, ferromagnetism, and nuclear magnetic resonance.
 W
WThe chemical shift index or CSI is a widely employed technique in protein nuclear magnetic resonance spectroscopy that can be used to display and identify the location as well as the type of protein secondary structure found in proteins using only backbone chemical shift data The technique was invented by Dr. David Wishart in 1992 for analyzing 1Hα chemical shifts and then later extended by him in 1994 to incorporate 13C backbone shifts. The original CSI method makes use of the fact that 1Hα chemical shifts of amino acid residues in helices tends to be shifted upfield relative to their random coil values and downfield in beta strands. Similar kinds of upfield/downfiled trends are also detectable in backbone 13C chemical shifts.
 W
WG. Marius Clore MAE, FRSC, FRS is a British-born, American molecular biophysicist and structural biologist. He was born in London, U.K. and is a dual US/U.K. Citizen. He is a member of the United States National Academy of Sciences, a Fellow of the Royal Society, a NIH Distinguished Investigator, and the Chief of the Protein NMR Spectroscopy Section in the Laboratory of Chemical Physics of the National Institute of Diabetes and Digestive and Kidney Diseases at the U.S. National Institutes of Health. He is known for his foundational work in three-dimensional protein and nucleic acid structure determination by biomolecular NMR spectroscopy, for advancing experimental approaches to the study of large macromolecules and their complexes by NMR, and for developing NMR-based methods to study rare conformational states in protein-nucleic acid and protein-protein recognition. Clore's discovery of previously undetectable, functionally significant, rare transient states of macromolecules has yielded fundamental new insights into the mechanisms of important biological processes, and in particular the significance of weak interactions and the mechanisms whereby the opposing constraints of speed and specificity are optimized. Further, Clore's work opens up a new era of pharmacology and drug design as it is now possible to target structures and conformations that have been heretofore unseen.
 W
WThe Collaborative Computing Project for NMR (CCPN) is a project that aims to bring together computational aspects of the scientific community involved in NMR spectroscopy, especially those who work in the field of protein NMR. The general aims are to link new and existing NMR software via a common data standard and provide a forum within the community for the discussion of NMR software and the scientific methods it supports. CCPN was initially started in 1999 in the United Kingdom but collaborates with NMR and software development groups worldwide.
 W
WRichard Robert Ernst is a Swiss physical chemist and Nobel Laureate.
 W
WFluorine-19 nuclear magnetic resonance spectroscopy is an analytical technique used to detect and identify fluorine-containing compounds. 19F is an important nucleus for NMR spectroscopy because of its receptivity and large chemical shift dispersion, which is greater than that for proton nuclear magnetic resonance spectroscopy.
 W
WIn Fourier transform nuclear magnetic resonance spectroscopy, free induction decay (FID) is the observable NMR signal generated by non-equilibrium nuclear spin magnetization precessing about the magnetic field. This non-equilibrium magnetization can be created generally by applying a pulse of radio-frequency close to the Larmor frequency of the nuclear spins.
 W
WThe Karplus equation, named after Martin Karplus, describes the correlation between 3J-coupling constants and dihedral torsion angles in nuclear magnetic resonance spectroscopy:
 W
WIn physics, Larmor precession is the precession of the magnetic moment of an object about an external magnetic field. Objects with a magnetic moment also have angular momentum and effective internal electric current proportional to their angular momentum; these include electrons, protons, other fermions, many atomic and nuclear systems, as well as classical macroscopic systems. The external magnetic field exerts a torque on the magnetic moment,
 W
WPaul Christian Lauterbur was an American chemist who shared the Nobel Prize in Physiology or Medicine in 2003 with Peter Mansfield for his work which made the development of magnetic resonance imaging (MRI) possible.
 W
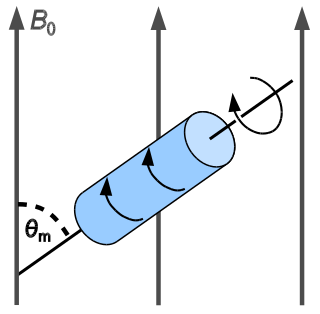
WIn nuclear magnetic resonance, magic-angle spinning (MAS) is a technique often used to perform experiments in solid-state NMR spectroscopy and, more recently, liquid Proton nuclear magnetic resonance.
 W
WA magnetometer is a device that measures magnetic field or magnetic dipole moment. Some magnetometers measure the direction, strength, or relative change of a magnetic field at a particular location. A compass is one such device, one that measures the direction of an ambient magnetic field, in this case, the Earth's magnetic field. Other magnetometers measure the magnetic dipole moment of a magnetic material such as a ferromagnet, for example by recording the effect of this magnetic dipole on the induced current in a coil.
 W
WSir Peter Mansfield was an English physicist who was awarded the 2003 Nobel Prize in Physiology or Medicine, shared with Paul Lauterbur, for discoveries concerning Magnetic Resonance Imaging (MRI). Mansfield was a professor at the University of Nottingham.
 W
WA microcoil is a tiny electrical conductor such as a wire in the shape of a spiral or helix which could be a solenoid or a planar structure. One field where these are found is nuclear magnetic resonance (NMR) spectroscopy, where it identifies radio frequency (RF) coils that are smaller than 1 mm.
 W
WAn MRI sequence in magnetic resonance imaging (MRI) is a particular setting of pulse sequences and pulsed field gradients, resulting in a particular image appearance.
 W
WAn NMR tube is a thin glass walled tube used to contain samples in nuclear magnetic resonance spectroscopy. Typically NMR tubes come in 5 mm diameters but 10 mm and 3 mm samples are known. It is important that the tubes are uniformly thick and well-balanced to ensure that NMR tube spins at a regular rate, usually about 20 Hz in the NMR spectrometer.
 W
WNuclear magnetic resonance quantum computing (NMRQC) is one of the several proposed approaches for constructing a quantum computer, that uses the spin states of nuclei within molecules as qubits. The quantum states are probed through the nuclear magnetic resonances, allowing the system to be implemented as a variation of nuclear magnetic resonance spectroscopy. NMR differs from other implementations of quantum computers in that it uses an ensemble of systems, in this case molecules, rather than a single pure state.
 W
WNuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds. Similarly, biochemists use NMR to identify proteins and other complex molecules. Besides identification, NMR spectroscopy provides detailed information about the structure, dynamics, reaction state, and chemical environment of molecules. The most common types of NMR are proton and carbon-13 NMR spectroscopy, but it is applicable to any kind of sample that contains nuclei possessing spin.
 W
WNuclear Medicine and Biology is a peer-reviewed medical journal published by Elsevier that covers research on all aspects of nuclear medicine, including radiopharmacology, radiopharmacy and clinical studies of targeted radiotracers. It is the official journal of the Society of Radiopharmaceutical Sciences. According to the Journal Citation Reports, the journal has a 2011 impact factor of 3.023.
 W
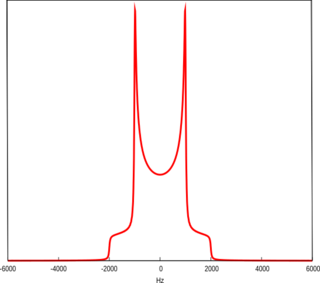
WA Pake Doublet is a characteristic line shape seen in solid-state nuclear magnetic resonance and electron paramagnetic resonance spectroscopy. It was first described by George Pake.
 W
WAlexander Pines is an American chemist. He is the Glenn T. Seaborg Professor Emeritus, University of California, Berkeley, Chancellor’s Professor Emeritus and Professor of the Graduate School, University of California, Berkeley, and a member of the California Institute for Quantitative Biosciences (QB3) and the Department of Bioengineering. He was born in 1945, grew up in Bulawayo in Southern Rhodesia and studied undergraduate mathematics and chemistry in Israel at Hebrew University of Jerusalem. Coming to the United States in 1968, Pines obtained his Ph.D. in chemical physics at M.I.T. in 1972 and joined the UC Berkeley faculty later that year.
 W
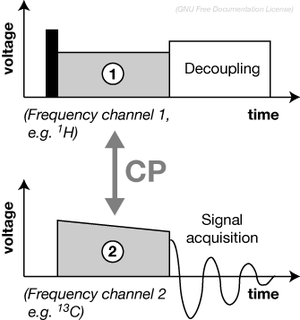
WProton-enhanced nuclear induction spectroscopy, also called cross-polarisation (CP), is a nuclear magnetic resonance technique invented by Michael Gibby and Alexander Pines while they were graduate students in the lab of Professor John S. Waugh at the Massachusetts Institute of Technology. Due to the suggestive nature of its acronym, the latter name is used more often. This technique is an integral part of most solid-state NMR experiments involving spin-1/2 nuclei.
 W
WIn Fourier transform NMR spectroscopy and imaging, a pulse sequence describes a series of radio frequency pulses applied to the sample, such that the free induction decay is related to the characteristic frequencies of the desired signals. After applying a Fourier transform, the signal can be represented in the frequency domain as the NMR spectrum. In magnetic resonance imaging, additional gradient pulses are applied by switching magnetic fields that exhibit a space-dependent gradient which can be used to reconstruct spatially resolved images after applying Fourier transforms.
 W
WEdward Mills Purcell was an American physicist who shared the 1952 Nobel Prize for Physics for his independent discovery of nuclear magnetic resonance in liquids and in solids. Nuclear magnetic resonance (NMR) has become widely used to study the molecular structure of pure materials and the composition of mixtures. Friends and colleagues knew him as Ed Purcell.
 W
WRandom coil index (RCI) predicts protein flexibility by calculating an inverse weighted average of backbone secondary chemical shifts and predicting values of model-free order parameters as well as per-residue RMSD of NMR and molecular dynamics ensembles from this parameter.
 W
WThe residual dipolar coupling between two spins in a molecule occurs if the molecules in solution exhibit a partial alignment leading to an incomplete averaging of spatially anisotropic dipolar couplings.
 W
WSolid-state NMR (ssNMR) spectroscopy is a special type of nuclear magnetic resonance (NMR) spectroscopy, characterized by the presence of anisotropic interactions. Compared to the more common solution NMR spectroscopy, ssNMR usually requires additional hardware for high-power radio-frequency irradiation and magic-angle spinning.
 W
WSolvent suppression is any technique in nuclear magnetic resonance spectroscopy (NMR) to decrease undesired signal from a sample's solvent.
 W
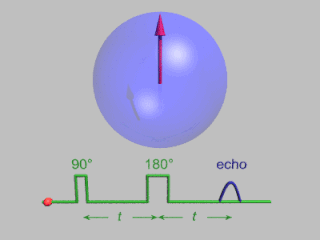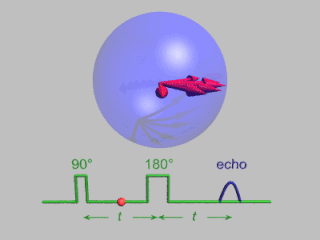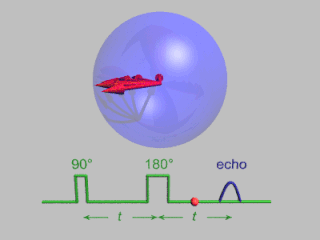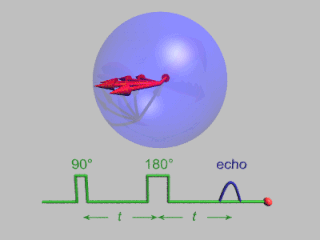
WIn magnetic resonance, a spin echo is the refocusing of spin magnetisation by a pulse of resonant electromagnetic radiation. Modern nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI) make use of this effect.
 W
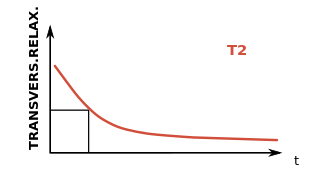
WIn physics, the spin–spin relaxation is the mechanism by which Mxy, the transverse component of the magnetization vector, exponentially decays towards its equilibrium value in nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI). It is characterized by the spin–spin relaxation time, known as T2, a time constant characterizing the signal decay. It is named in contrast to T1, the spin–lattice relaxation time. It is the time it takes for the magnetic resonance signal to irreversibly decay to 37% (1/e) of its initial value after its generation by tipping the longitudinal magnetization towards the magnetic transverse plane. Hence the relation.
 W
WSpinlock is a technology based company specialized in the manufacture and development of nuclear magnetic resonance (NMR) and nuclear quadrupole resonance (NQR) equipment.
 W
WTributylammonium TRISPHAT is an organic salt with the formula [(C4H9)3NH+][P(O2C6Cl4)−3]. The anion features phosphorus(V) bonded to three tetrachlorocatecholate ligands. This anion can be resolved into the enantiomers, which are optically stable.
 W
WKurt Wüthrich is a Swiss chemist/biophysicist and Nobel Chemistry laureate, known for developing nuclear magnetic resonance (NMR) methods for studying biological macromolecules.