 W
WPressure is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure is the pressure relative to the ambient pressure.
 W
WBlood pressure (BP) is the pressure of circulating blood against the walls of blood vessels. Most of this pressure results from the heart pumping blood through the circulatory system. When used without qualification, the term "blood pressure" refers to the pressure in the large arteries. Blood pressure is usually expressed in terms of the systolic pressure over diastolic pressure in the cardiac cycle. It is measured in millimeters of mercury (mmHg) above the surrounding atmospheric pressure.
 W
WCold inflation pressure is the inflation pressure of tires before the car is driven and the tires(tyres) warmed up. Recommended cold inflation pressure is displayed on the owner's manual and on the placard attached to the vehicle door edge, pillar, glovebox door or fuel filler flap. 40% of passenger cars have at least one tyre under-inflated by 6 psi or more. Drivers are encouraged to make sure their tires(tyres) are adequately inflated, as under inflated tires(tyres) can greatly reduce fuel economy, increase emissions, cause increased wear on the edges of the tread surface, and can lead to overheating and premature failure of the tire(tyre). Excessive pressure, on the other hand, may lead to impact-breaks, decreased braking performance, and cause increased wear on the center part of the tread surface.
 W
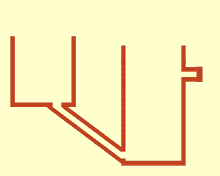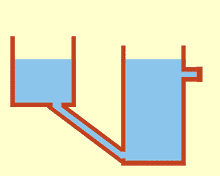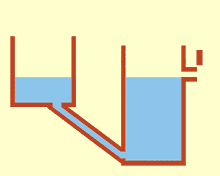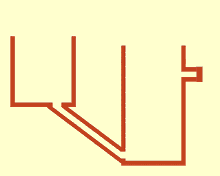
WCommunicating vessels is a system of containers filled with a homogeneous fluid, connected at the base and subjected to the same atmospheric pressure. When the liquid settles, it balances out to the same level in all of the containers regardless of their shape and volume. If additional liquid is added to one vessel, a new equal level will be established in all the connected vessels. This process is part of Stevin's Law and occurs because gravity and pressure are constant in each vessel.
 W
WA filtered air positive pressure environment in laboratory animal science is a space that is under positive pressure with respect to the outside world. In this way, no germs that could affect the lab animals or that are a threat to the SPF status can enter the facility. To bring in new air, high efficiency particulate air filters (HEPA) are used.
 W
WFluid statics or hydrostatics is the branch of fluid mechanics that studies "fluids at rest and the pressure in a fluid or exerted by a fluid on an immersed body".
 W
WThe Laplace pressure is the pressure difference between the inside and the outside of a curved surface that forms the boundary between a gas region and a liquid region. The pressure difference is caused by the surface tension of the interface between liquid and gas.
 W
WNegative room pressure is an isolation technique used in hospitals and medical centers to prevent cross-contamination from room to room. It includes a ventilation that generates "negative pressure" to allow air to flow into the isolation room but not escape from the room, as air will naturally flow from areas with higher pressure to areas with lower pressure, thereby preventing contaminated air from escaping the room. This technique is used to isolate patients with airborne contagious diseases such as: influenza (flu), measles, chickenpox, tuberculosis, Severe Acute Respiratory Syndrome (SARS-CoV), Middle East Respiratory Syndrome (MERS-CoV), and Coronavirus disease 2019 (COVID-19).
 W
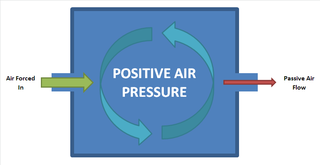
WPositive pressure is a pressure within a system that is greater than the environment that surrounds that system. Consequently, if there is any leak from the positively pressured system it will egress into the surrounding environment. This is in contrast to a negative pressure room, where air is sucked in.
 W
WPressure cooking is the process of cooking food under high pressure steam, employing water or a water-based cooking liquid, in a sealed vessel known as a pressure cooker. High pressure limits boiling, and permits cooking temperatures well above 100 °C (212 °F) to be reached.
 W
WA pressure sensor is a device for pressure measurement of gases or liquids. Pressure is an expression of the force required to stop a fluid from expanding, and is usually stated in terms of force per unit area. A pressure sensor usually acts as a transducer; it generates a signal as a function of the pressure imposed. For the purposes of this article, such a signal is electrical.
 W
WPressurisation duct work is a passive fire protection system. It is used to supply fresh air to any area of refuge, designated emergency evacuation or egress route.
 W
WRam pressure is a pressure exerted on a body moving through a fluid medium, caused by relative bulk motion of the fluid rather than random thermal motion. It causes a drag force to be exerted on the body. Ram pressure is given in tensor form as
 W
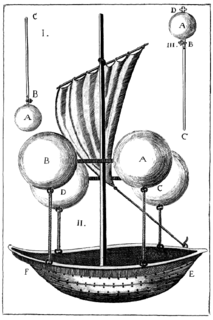
WTorricelli's experiment was invented in Pisa in 1643 by the Italian scientist Evangelista Torricelli (1608-1647). The purpose of his experiment is to prove that the source of vacuum comes from atmospheric pressure.
 W
WA vacuum airship, also known as a vacuum balloon, is a hypothetical airship that is evacuated rather than filled with a lighter-than-air gas such as hydrogen or helium. First proposed by Italian Jesuit priest Francesco Lana de Terzi in 1670, the vacuum balloon would be the ultimate expression of lifting power per volume displaced.
 W
WIn physics, a vapor or vapour is a substance in the gas phase at a temperature lower than its critical temperature, which means that the vapor can be condensed to a liquid by increasing the pressure on it without reducing the temperature. A vapor is different from an aerosol. An aerosol is a suspension of tiny particles of liquid, solid, or both within a gas.
 W
WVapor pressure or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's evaporation rate. It relates to the tendency of particles to escape from the liquid. A substance with a high vapor pressure at normal temperatures is often referred to as volatile. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the kinetic energy of its molecules also increases. As the kinetic energy of the molecules increases, the number of molecules transitioning into a vapor also increases, thereby increasing the vapor pressure.