 W
WTemperature is a physical quantity that expresses hot and cold. It is the manifestation of thermal energy, present in all matter, which is the source of the occurrence of heat, a flow of energy, when a body is in contact with another that is colder or hotter.
 W
WAbsolute zero is the lowest limit of the thermodynamic temperature scale, a state at which the enthalpy and entropy of a cooled ideal gas reach their minimum value, taken as zero kelvins. The fundamental particles of nature have minimum vibrational motion, retaining only quantum mechanical, zero-point energy-induced particle motion. The theoretical temperature is determined by extrapolating the ideal gas law; by international agreement, absolute zero is taken as −273.15° on the Celsius scale, which equals −459.67° on the Fahrenheit scale. The corresponding Kelvin and Rankine temperature scales set their zero points at absolute zero by definition.
 W
WAtmospheric temperature is a measure of temperature at different levels of the Earth's atmosphere. It is governed by many factors, including incoming solar radiation, humidity and altitude. When discussing surface air temperature, the annual atmospheric temperature range at any geographical location depends largely upon the type of biome, as measured by the Köppen climate classification
 W
WThe building balance point temperature is the outdoor air temperature when the heat gains of the building are equal to the heat losses. Internal heat sources due to electric lighting, mechanical equipment, body heat, and solar radiation may offset the need for additional heating although the outdoor temperature may be below the thermostat set-point temperature. The building balance point temperature is the base temperature necessary to calculate heating degree day to anticipate the annual energy demand to heat a building. The balance point temperature is a consequence of building design and function rather than outdoor weather conditions.
 W
WThe boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
 W
WIn thermodynamics, the bubble point is the temperature where the first bubble of vapor is formed when heating a liquid consisting of two or more components. Given that vapor will probably have a different composition than the liquid, the bubble point at different compositions are useful data when designing distillation systems.
 W
WIn physics and materials science, the Curie temperature (TC), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can be replaced by induced magnetism. The Curie temperature is named after Pierre Curie, who showed that magnetism was lost at a critical temperature.
 W
WThe dew point is the temperature to which air must be cooled to become saturated with water vapor. When cooled further, the airborne water vapor will condense to form liquid water (dew). When air cools to its dew point through contact with a surface that is colder than the air, water will condense on the surface.
 W
WA thermometer is a device that measures temperature or a temperature gradient. A thermometer has two important elements: (1) a temperature sensor in which some change occurs with a change in temperature; and (2) some means of converting this change into a numerical value. Thermometers are widely used in technology and industry to monitor processes, in meteorology, in medicine, and in scientific research.
 W
WThe global temperature record shows the fluctuations of the temperature of the atmosphere and the oceans through various spans of time. The most detailed information exists since 1850, when methodical thermometer-based records began. There are numerous estimates of temperatures since the end of the Pleistocene glaciation, particularly during the current Holocene epoch. Older time periods are studied by paleoclimatology.
 W
WThe lapse rate is the rate at which an atmospheric variable, normally temperature in Earth's atmosphere, falls with altitude. Lapse rate arises from the word lapse, in the sense of a gradual fall.
 W
WCertain systems can achieve negative thermodynamic temperature; that is, their temperature can be expressed as a negative quantity on the Kelvin or Rankine scales. This should be distinguished from temperatures expressed as negative numbers on non-thermodynamic Celsius or Fahrenheit scales, which are nevertheless higher than absolute zero.
 W
WThe neutron detection temperature, also called the neutron energy, indicates a free neutron's kinetic energy, usually given in electron volts. The term temperature is used, since hot, thermal and cold neutrons are moderated in a medium with a certain temperature. The neutron energy distribution is then adapted to the Maxwellian distribution known for thermal motion. Qualitatively, the higher the temperature, the higher the kinetic energy of the free neutrons. The momentum and wavelength of the neutron are related through the de Broglie relation. The large wavelength of slow neutrons allows for the large cross section.
 W
WTemperature measurement describes the process of measuring a current local temperature for immediate or later evaluation. Datasets consisting of repeated standardized measurements can be used to assess temperature trends.
 W
WPyrometric cones are pyrometric devices that are used to gauge heatwork during the firing of ceramic materials. The cones, often used in sets of three, are positioned in a kiln with the wares to be fired and provide a visual indication of when the wares have reached a required state of maturity, a combination of time and temperature. Thus, pyrometric cones give a temperature equivalent; they are not simple temperature-measuring devices.
 W
WThe practice of using colours to determine the temperature of a piece of (usually) ferrous metal comes from blacksmithing. Long before thermometers were widely available it was necessary to know what state the metal was in for heat treating it and the only way to do this was to heat it up to a colour which was known to be best for the work.
 W
WColloquially, room temperature is the range of air temperatures that most people prefer for indoor settings, which feel comfortable when wearing typical indoor clothing. Human comfort can extend beyond this range depending on humidity, air circulation and other factors. In certain fields, like science and engineering, and within a particular context, room temperature can mean different agreed-on ranges. In contrast, ambient temperature is the actual temperature of the air in any particular place, as measured by a thermometer. It may be very different from usual room temperature, for example an unheated room in winter.
 W
WSatellite temperature measurements are inferences of the temperature of the atmosphere at various altitudes as well as sea and land surface temperatures obtained from radiometric measurements by satellites. These measurements can be used to locate weather fronts, monitor the El Niño-Southern Oscillation, determine the strength of tropical cyclones, study urban heat islands and monitor the global climate. Wildfires, volcanos, and industrial hot spots can also be found via thermal imaging from weather satellites.
 W
WIn oceanography, temperature-salinity diagrams, sometimes called T-S diagrams, are used to identify water masses. In a T-S diagram, rather than plotting each water property as a separate "profile," with pressure or depth as the vertical coordinate, potential temperature is plotted versus salinity. As long as it remains isolated from the surface, where heat or fresh water can be gained or lost, and in the absence of mixing with other water masses, a water parcel's potential temperature and salinity are conserved. Deep water masses thus retain their T-S characteristics for long periods of time, and can be identified readily on a T-S plot.
 W
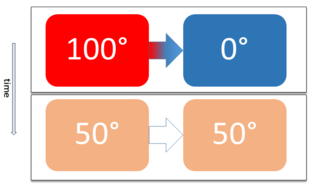
WTwo physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be in thermal equilibrium with itself if the temperature within the system is spatially uniform and temporally constant.
 W
WThermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics.
 W
WA thermometer is a device that measures temperature or a temperature gradient. A thermometer has two important elements: (1) a temperature sensor in which some change occurs with a change in temperature; and (2) some means of converting this change into a numerical value. Thermometers are widely used in technology and industry to monitor processes, in meteorology, in medicine, and in scientific research.
 W
WThe wet-bulb globe temperature (WBGT) is a type of apparent temperature used to estimate the effect of temperature, humidity, wind speed, and visible and infrared radiation on humans. It is used by industrial hygienists, athletes, sporting events and the military to determine appropriate exposure levels to high temperatures. It is derived from the following formula:
 W
WThe wet-bulb temperature (WBT) is the temperature read by a thermometer covered in water-soaked cloth over which air is passed. At 100% relative humidity, the wet-bulb temperature is equal to the air temperature ; at lower humidity the wet-bulb temperature is lower than dry-bulb temperature because of evaporative cooling.