 W
WA fuel cell is an electrochemical cell that converts the chemical energy of a fuel and an oxidizing agent into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from metals and their ions or oxides that are commonly already present in the battery, except in flow batteries. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied.
 W
WAn alkaline anion exchange membrane fuel cell (AAEMFC), also known as anion-exchange membrane fuel cells (AEMFCs), alkaline membrane fuel cells (AMFCs), hydroxide exchange membrane fuel cells (HEMFCs), or solid alkaline fuel cells (SAFCs) is a type of alkaline fuel cell that uses an anion exchange membrane to separate the anode and cathode compartments.
 W
WThe alkaline fuel cell (AFC), also known as the Bacon fuel cell after its British inventor, Francis Thomas Bacon, is one of the most developed fuel cell technologies. Alkaline fuel cells consume hydrogen and pure oxygen, to produce potable water, heat, and electricity. They are among the most efficient fuel cells, having the potential to reach 70%.
 W
WDr. Bernard S. Baker was born in Philadelphia and a resident of Bethel, Connecticut in the United States. He was a pioneer in the field of electrochemistry and his career spanned 45 years. He was a founder and served as president, chief executive officer and chairman of Energy Research Corporation, developer and manufacturer of direct fuel cells (MCFC) used to generate electric power. Power plants based on his concepts are providing electricity in distributed generation locations throughout the world.
 W
WThe Bloom Energy Server or Bloom Box is a solid oxide fuel cell (SOFC) power generator made by Bloom Energy, of Sunnyvale, California, that takes a variety of input fuels, including liquid or gaseous hydrocarbons produced from biological sources, to produce electricity at or near the site where it will be used. It withstands temperatures of up to 1,800 °F (980 °C). According to the company, a single cell generates 25 watts.
 W
WChemSusChem is a top interdisciplinary peer-reviewed scientific journal founded in 2008 and published by Wiley-VCH on behalf of Chemistry Europe and is a sister publication to other scientific journals published by Wiley-VCH, including Angewandte Chemie and Chemistry—A European Journal. The journal publishes a wide range of research at the interface of chemistry and sustainability, including contributions from chemistry, materials science, chemical engineering, and biotechnology through a mix of full papers, communications, reviews, concepts, highlights, and viewpoints.
 W
WDirect-methanol fuel cells or DMFCs are a subcategory of proton-exchange fuel cells in which methanol is used as the fuel. Their main advantage is the ease of transport of methanol, an energy-dense yet reasonably stable liquid at all environmental conditions.
 W
WNedjib (Ned) Djilali is a Canadian engineering professor and researcher specializing in sustainable energy and thermofluid sciences. He holds the Canada Research Chair in Advanced Energy Systems Design and Computational Modelling at the University of Victoria. Djilali is a Highly Cited Researcher, and a fellow of both the Canadian Academy of Engineering (2010) and the Royal Society of Canada (2013).
 W
WAn electro-galvanic fuel cell is an electrochemical device which consumes a fuel to produce an electrical output by a chemical reaction. One form of electro-galvanic fuel cell based on the oxidation of lead is commonly used to measure the concentration of oxygen gas in underwater diving and medical breathing gases.
 W
WA flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion exchange occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts.
 W
WA membrane electrode assembly (MEA) is an assembled stack of proton exchange membranes (PEM) or alkali anion exchange membrane (AAEM), catalyst and flat plate electrode used in fuel cells and electrolyzers.
 W
WMetal hydride fuel cells are a subclass of alkaline fuel cells that have been under research and development, as well as scaled up successfully in operating systems. A notable feature is their ability to chemically bond and store hydrogen within the fuel cell itself.
 W
WA microbial electrolysis cell (MEC) is a technology related to Microbial fuel cells (MFC). Whilst MFCs produce an electric current from the microbial decomposition of organic compounds, MECs partially reverse the process to generate hydrogen or methane from organic material by applying an electric current. The electric current would ideally be produced by a renewable source of power. The hydrogen or methane produced can be used to produce electricity by means of an additional PEM fuel cell or internal combustion engine.
 W
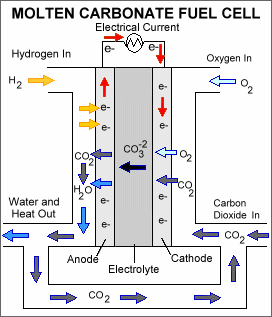
WMolten-carbonate fuel cells (MCFCs) are high-temperature fuel cells that operate at temperatures of 600 °C and above.
 W
WOsmotic power, salinity gradient power or blue energy is the energy available from the difference in the salt concentration between seawater and river water. Two practical methods for this are reverse electrodialysis (RED) and pressure retarded osmosis (PRO). Both processes rely on osmosis with membranes. The key waste product is brackish water. This byproduct is the result of natural forces that are being harnessed: the flow of fresh water into seas that are made up of salt water.
 W
WPhosphoric acid fuel cells (PAFC) are a type of fuel cell that uses liquid phosphoric acid as an electrolyte. They were the first fuel cells to be commercialized. Developed in the mid-1960s and field-tested since the 1970s, they have improved significantly in stability, performance, and cost. Such characteristics have made the PAFC a good candidate for early stationary applications.
 W
WPressure retarded osmosis (PRO) is a technique to separate a solvent from a solution that is more concentrated and also pressurized. A semipermeable membrane allows the solvent to pass to the concentrated solution side by osmosis. The technique can be used to generate power from the salinity gradient energy resulting from the difference in the salt concentration between sea and river water. In PRO, the water potential between fresh water and sea water corresponds to a pressure of 26 bars. This pressure is equivalent to a column of water 270 meters high. However, the optimal working pressure is only half of this, 11 to 15 bar.
 W
WProton-exchange membrane fuel cells (PEMFC), also known as polymer electrolyte membrane (PEM) fuel cells, are a type of fuel cell being developed mainly for transport applications, as well as for stationary fuel-cell applications and portable fuel-cell applications. Their distinguishing features include lower temperature/pressure ranges and a special proton-conducting polymer electrolyte membrane. PEMFCs generate electricity and operate on the opposite principle to PEM electrolysis, which consumes electricity. They are a leading candidate to replace the aging alkaline fuel-cell technology, which was used in the Space Shuttle.
 W
WA protonic ceramic fuel cell or PCFC is a fuel cell based around a ceramic electrolyte material that exhibits high protonic conductivity at elevated temperatures.
 W
WThe PureCell System is a stationary phosphoric acid fuel cell designed, manufactured and marketed by Doosan Fuel Cell America of South Windsor, Connecticut. Intended for distributed generation and micro combined heat and power applications, it is considered a good match for commercial and industrial buildings such as hotels, hospitals, data centers, supermarkets and educational institutions. PureCell System says that its users will see lower energy costs, reduced emissions, 95% system efficiency, 10-year cell stack durability and 20-year product life. It utilizes a combustion-free process with natural gas and converts heat exhaust into cooling and heating, turning potential waste into usable energy.
 W
WReformed Methanol Fuel Cell (RMFC) or Indirect Methanol Fuel Cell (IMFC) systems are a subcategory of proton-exchange fuel cells where, the fuel, methanol (CH3OH), is reformed, before being fed into the fuel cell. RMFC systems offer advantages over direct methanol fuel cell (DMFC) systems including higher efficiency, smaller cell stacks, no water management, better operation at low temperatures, and storage at sub-zero temperatures because methanol is a liquid from -97.0 °C to 64.7 °C (-142.6 °F to 148.5 °F). The tradeoff is that RMFC systems operate at hotter temperatures and therefore need more advanced heat management and insulation. The waste products with these types of fuel cells are carbon dioxide and water.
 W
WA solid oxide electrolyzer cell (SOEC) is a solid oxide fuel cell that runs in regenerative mode to achieve the electrolysis of water by using a solid oxide, or ceramic, electrolyte to produce hydrogen gas and oxygen. The production of pure hydrogen is compelling because it is a clean fuel that can be stored easily, thus making it a potential alternative to batteries, which have a low storage capacity and create high amounts of waste materials. Electrolysis is currently the most promising method of hydrogen production from water due to high efficiency of conversion and relatively low required energy input when compared to thermochemical and photocatalytic methods.
 W
WA solid oxide fuel cell is an electrochemical conversion device that produces electricity directly from oxidizing a fuel. Fuel cells are characterized by their electrolyte material; the SOFC has a solid oxide or ceramic electrolyte.
 W
WThe principle of a fuel cell was discovered by Christian Friedrich Schönbein in 1838, and the first fuel cell was constructed by Sir William Robert Grove in 1839. The fuel cells made at this time were most similar to today's phosphoric acid fuel cells. Most hydrogen fuel cells today are of the proton exchange membrane (PEM) type. A PEM converts the chemical energy released during the electrochemical reaction of hydrogen and oxygen into electrical energy. The Energy Policy Act of 1992 was the first national legislation that called for large-scale hydrogen research. A five-year program was conducted that investigated the production of hydrogen from renewable energy sources and the feasibility of existing natural gas pipelines to carry hydrogen. It also called for the research into hydrogen storage systems for electric vehicles and the development of fuel cells suitable to power an electric motor vehicle.
 W
WWoking Park is a large park and leisure complex in Woking, Surrey, operated and maintained by Woking Borough Council. The park is in the Hoe Valley and will be affected by the Hoe Valley Scheme.
 W
WZinc–air batteries (non-rechargeable), and zinc–air fuel cells are metal–air batteries powered by oxidizing zinc with oxygen from the air. These batteries have high energy densities and are relatively inexpensive to produce. Sizes range from very small button cells for hearing aids, larger batteries used in film cameras that previously used mercury batteries, to very large batteries used for electric vehicle propulsion and grid-scale energy storage.