 W
WPhotochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet, visible light (400–750 nm) or infrared radiation (750–2500 nm).
 W
W3D optical data storage is any form of optical data storage in which information can be recorded or read with three-dimensional resolution.
 W
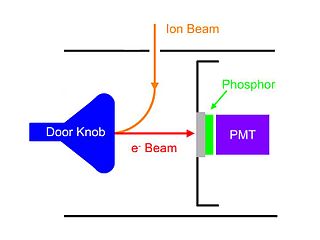
WA Daly detector is a gas-phase ion detector that consists of a metal "doorknob", a scintillator and a photomultiplier. It was named after its inventor Norman Richard Daly. Daly detectors are typically used in mass spectrometers.
 W
WAn excimer is a short-lived dimeric or heterodimeric molecule formed from two species, at least one of which has a valence shell completely filled with electrons. In this case, formation of molecules is possible only if such atom is in an electronic excited state. Heteronuclear molecules and molecules that have more than two species are also called exciplex molecules. Excimers are often diatomic and are composed of two atoms or molecules that would not bond if both were in the ground state. The lifetime of an excimer is very short, on the order of nanoseconds. Binding of a larger number of excited atoms forms Rydberg matter clusters, the lifetime of which can exceed many seconds.
 W
WFemtochemistry is the area of physical chemistry that studies chemical reactions on extremely short timescales in order to study the very act of atoms within molecules (reactants) rearranging themselves to form new molecules (products). In a 1988 issue of the journal Science, Ahmed Hassan Zewail published an article using this term for the first time, stating "Real-time femtochemistry, that is, chemistry on the femtosecond timescale...". Later in 1999, Zewail received the Nobel Prize in Chemistry for his pioneering work in this field showing that it is possible to see how atoms in a molecule move during a chemical reaction with flashes of laser light.
 W
WA Grotrian diagram, or term diagram, shows the allowed electronic transitions between the energy levels of atoms. They can be used for one-electron and multi-electron atoms. They take into account the specific selection rules related to changes in angular momentum of the electron. The diagrams are named after Walter Grotrian, who introduced them in his 1928 book Graphische Darstellung der Spektren von Atomen und Ionen mit ein, zwei und drei Valenzelektronen.
 W
WIndirect DNA damage occurs when a UV-photon is absorbed in the human skin by a chromophore that does not have the ability to convert the energy into harmless heat very quickly. Molecules that do not have this ability have a long-lived excited state. This long lifetime leads to a high probability for reactions with other molecules—so-called bimolecular reactions. Melanin and DNA have extremely short excited state lifetimes in the range of a few femtoseconds (10−15s). The excited state lifetime of compounds used in sunscreens such as menthyl anthranilate, avobenzone or padimate O is 1,000 to 1,000,000 times longer than that of melanin, and therefore they may cause damage to living cells that come in contact with them.
 W
WInternal conversion is a transition from a higher to a lower electronic state in a molecule or atom. It is sometimes called "radiationless de-excitation", because no photons are emitted. It differs from intersystem crossing in that, while both are radiationless methods of de-excitation, the molecular spin state for internal conversion remains the same, whereas it changes for intersystem crossing. The energy of the electronically excited state is given off to vibrational modes of the molecule. The excitation energy is transformed into heat.
 W
WIn molecular spectroscopy, a Jablonski diagram is a diagram that illustrates the electronic states of a molecule and the transitions between them. The states are arranged vertically by energy and grouped horizontally by spin multiplicity. Nonradiative transitions are indicated by squiggly arrows and radiative transitions by straight arrows. The vibrational ground states of each electronic state are indicated with thick lines, the higher vibrational states with thinner lines. The diagram is named after the Polish physicist Aleksander Jabłoński.
 W
WLightfastness is a property of a colourant such as dye or pigment that describes how resistant to fading it is when exposed to light. Dyes and pigments are used for example for dyeing of fabrics, plastics or other materials and manufacturing paints or printing inks.
 W
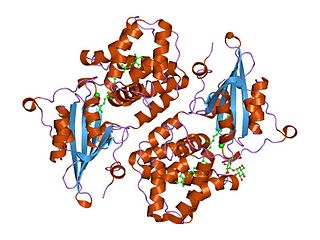
WOrange carotenoid protein (OCP) is a water-soluble protein which plays a role in photoprotection in diverse cyanobacteria. It is the only photoactive protein known to use a carotenoid as the photoresponsive chromophore. The protein consists of two domains, with a single keto-carotenoid molecule non-covalently bound between the two domains. It is a very efficient quencher of excitation energy absorbed by the primary light-harvesting antenna complexes of cyanobacteria, the phycobilisomes. The quenching is induced by blue-green light. It is also capable of preventing oxidative damage by directly scavenging singlet oxygen (1O2).
 W
WOtto Perutz was an Austrian-German chemist.
 W
W2-Phenylpyridine is an organic compound with the formula C6H5C5H4N (or C11H9N). It is a colourless viscous liquid. The compound and related derivatives have attracted interest as precursors to highly fluorescent metal complexes of possible value as organic light emitting diodes (OLEDs).
 W
Wl-Photo-leucine is a synthetic derivative of the l-leucine amino acid that is used as its natural analog and is characterized for having photo-reactivity, which makes it suitable for observing and characterizing protein-protein interactions (PPI). When a protein containing this amino acid (A) is lightened with ultraviolet light while interacting with another protein (B), the complex formed from these two proteins (AB) remains attached and can be isolated for its study.
 W
WIn chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a catalyst. In catalysed photolysis, light is absorbed by an adsorbed substrate. In photogenerated catalysis, the photocatalytic activity (PCA) depends on the ability of the catalyst to create electron–hole pairs, which generate free radicals (e.g. hydroxyl radicals: •OH) able to undergo secondary reactions. Its practical application was made possible by the discovery of water electrolysis by means of titanium dioxide (TiO2).
 W
WPhotochromism is the reversible transformation of a chemical species between two forms by the absorption of electromagnetic radiation (photoisomerization), where the two forms have different absorption spectra. In plain language, this can be described as a reversible change of colour upon exposure to light.
 W
WPhotodegradation is the alteration of materials by light. Typically, the term refers to the combined action of sunlight and air. Photodegradation is usually oxidation and hydrolysis. Often photodegradation is avoided, since it destroys paintings and other artifacts. It is however partly responsible for remineralization of biomass and is used intentionally in some disinfection technologies. Photodegradation does not apply to how materials may be aged or degraded via infrared light or heat, but does include degradation in all of the ultraviolet light wavebands.
 W
WThe photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, and solid state and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission.
 W
WPhotogeochemistry merges photochemistry and geochemistry into the study of light-induced chemical reactions that occur or may occur among natural components of Earth's surface. The first comprehensive review on the subject was published in 2017 by the chemist and soil scientist Timothy A Doane, but the term photogeochemistry appeared a few years earlier as a keyword in studies that described the role of light-induced mineral transformations in shaping the biogeochemistry of Earth; this indeed describes the core of photogeochemical study, although other facets may be admitted into the definition.
 W
WPhotoinduced electron transfer (PET) is an excited state electron transfer process by which an excited electron is transferred from donor to acceptor. Due to PET a charge separation is generated, i.e., redox reaction takes place in excited state.
 W
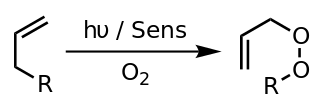
WA photooxygenation is a light-induced oxidation reaction in which molecular oxygen is incorporated into the product(s). Initial research interest in photooxygenation reactions arose from Oscar Raab's observations in 1900 that the combination of light, oxygen and photosensitizers is highly toxic to cells. Early studies of photooxygenation focused on oxidative damage to DNA and amino acids, but recent research has led to the application of photooxygenation in organic synthesis and photodynamic therapy.
 W
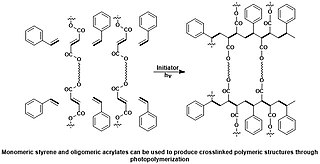
WA photopolymer or light-activated resin is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing.
 W
WPhotoredox catalysis is a branch of catalysis that harnesses the energy of light to accelerate a chemical reaction via single-electron transfer events. This area is named as a combination of "photo-" referring to light and redox, a condensed expression for the chemical processes of reduction and oxidation. In particular, photoredox catalysis employs small quantities of a light-sensitive compound that, when excited by light, can mediate the transfer of electrons between chemical compounds that would usually not react at all. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes, and semiconductors. While organic photoredox catalysts were dominant throughout the 1990s and early 2000s, soluble transition-metal complexes are more commonly used today.
 W
WA photosensitizer is a molecule that produces a chemical change in another molecule in a photochemical process. Photosensitizers are commonly used in polymer chemistry in reactions such as photopolymerization, photocrosslinking, and photodegradation. Photosensitizers are also used to generate triplet excited states in organic molecules with uses in photocatalysis, photon upconversion and photodynamic therapy. Photosensitizers generally act by absorbing ultraviolet or visible region of electromagnetic radiation and transferring it to adjacent molecules. Photosensitizers usually have large de-localized π systems, which lower the energy of HOMO orbitals and its absorption of light might be able to ionize the molecule. There are also examples of using semiconductor quantum dots as photosensitizers.
 W
WQuantum photoelectrochemistry is the investigation of the quantum mechanical nature of photoelectrochemistry, the subfield of study within physical chemistry concerned with the interaction of light with electrochemical systems, typically through the application of quantum chemical calculations. Quantum photoelectrochemistry provides an expansion of quantum electrochemistry to processes involving also the interaction with light (photons). It therefore also includes essential elements of photochemistry. Key aspects of quantum photoelectrochemistry are calculations of optical excitations, photoinduced electron and energy transfer processes, excited state evolution, as well as interfacial charge separation and charge transport in nanoscale energy conversion systems.
 W
WA scintillator is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate. Sometimes, the excited state is metastable, so the relaxation back down from the excited state to lower states is delayed. The process then corresponds to one of two phenomena: delayed fluorescence or phosphorescence. The correspondence depends on the type of transition and hence the wavelength of the emitted optical photon.
 W
WSmall molecule sensors are an effective way to detect the presence of metal ions in solution. Although many types exist, most small molecule sensors comprise a subunit that selectively binds to a metal that in turn induces a change in a fluorescent subunit. This change can be observed in the small molecule sensor's spectrum, which can be monitored using a detection system such as a microscope or a photodiode. Different probes exist for a variety of applications, each with different dissociation constants with respect to a particular metal, different fluorescent properties, and sensitivities. They show great promise as a way to probe biological processes by monitoring metal ions at low concentrations in biological systems. Since they are by definition small and often capable of entering biological systems, they are conducive to many applications for which other more traditional bio-sensing are less effective or not suitable.
 W
WSolar energy conversion describes technologies devoted to the transformation of solar energy to other (useful) forms of energy, including electricity, fuel, and heat. It covers light-harvesting technologies including traditional semiconductor photovoltaic devices (PVs), emerging photovoltaics, solar fuel generation via electrolysis, artificial photosynthesis, and related forms of photocatalysis directed at the generation of energy rich molecules.
 W
WA solar hydrogen panel is a device for artificial photosynthesis that produces hydrogen directly from sunlight and water vapor utilizing phytocatalytic water splitting and thus bypasses the conversion losses of the classical solar–hydrogen energy cycle where solar power is first harvested with solar panels and only then to converted to hydrogen with electrolysis plants.
 W
WSpectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. Spectrophotometry uses photometers, known as spectrophotometers, that can measure the intensity of a light beam at different wavelengths. Although spectrophotometry is most commonly applied to ultraviolet, visible, and infrared radiation, modern spectrophotometers can interrogate wide swaths of the electromagnetic spectrum, including x-ray, ultraviolet, visible, infrared, and/or microwave wavelengths.
 W
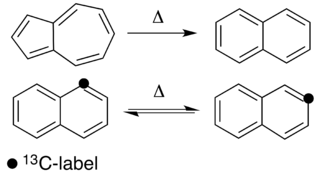
WThermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (isomerization), and one in which the atoms are scrambled but no net change in the aromatic ring occurs (automerization). The general reaction schemes of the two types are illustrated in Figure 1.
 W
WThe transition dipole moment or transition moment, usually denoted for a transition between an initial state, , and a final state, , is the electric dipole moment associated with the transition between the two states. In general the transition dipole moment is a complex vector quantity that includes the phase factors associated with the two states. Its direction gives the polarization of the transition, which determines how the system will interact with an electromagnetic wave of a given polarization, while the square of the magnitude gives the strength of the interaction due to the distribution of charge within the system. The SI unit of the transition dipole moment is the Coulomb-meter (Cm); a more conveniently sized unit is the Debye (D).
 W
WTriboluminescence is an optical phenomenon in which light is generated when a material is mechanically pulled apart, ripped, scratched, crushed, or rubbed. The phenomenon is not fully understood, but appears to be caused by the separation and reunification of static electrical charges. The term comes from the Greek τρίβειν and the Latin lumen (light). Triboluminescence can be observed when breaking sugar crystals and peeling adhesive tapes.
 W
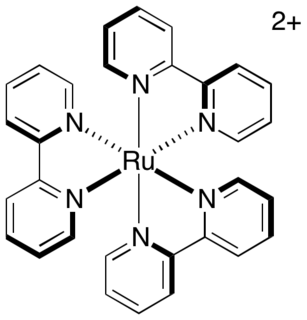
WTris(bipyridine)ruthenium(II) chloride is the chloride salt coordination complex with the formula [Ru(bpy)3]2+. This red crystalline salt is obtained as the hexahydrate, although all of the properties of interest are in the cation [Ru(bpy)3]2+, which has received much attention because of its distinctive optical properties. The chlorides can be replaced with other anions, such as PF6−.
 W
WHoward E. Zimmerman aka Z was a professor of chemistry at the University of Wisconsin–Madison. He was elected to the National Academy of Sciences in 1980 and the recipient of the 1986 American Institute of Chemists Chemical Pioneer Award.