 W
WLuminescence is spontaneous emission of light by a substance not resulting from heat; or "cold light".
 W
WAn anode ray is a beam of positive ions that is created by certain types of gas-discharge tubes. They were first observed in Crookes tubes during experiments by the German scientist Eugen Goldstein, in 1886. Later work on anode rays by Wilhelm Wien and J. J. Thomson led to the development of mass spectrometry.
 W
WA blacklight, also referred to as a UV-A light, Wood's lamp, or ultraviolet light, is a lamp that emits long-wave (UV-A) ultraviolet light and very little visible light.
 W
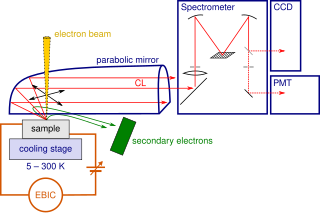
WCathodoluminescence is an optical and electromagnetic phenomenon in which electrons impacting on a luminescent material such as a phosphor, cause the emission of photons which may have wavelengths in the visible spectrum. A familiar example is the generation of light by an electron beam scanning the phosphor-coated inner surface of the screen of a television that uses a cathode ray tube. Cathodoluminescence is the inverse of the photoelectric effect, in which electron emission is induced by irradiation with photons.
 W
WChemiluminescence is the emission of light (luminescence), as the result of a chemical reaction. There may also be limited emission of heat. Given reactants A and B, with an excited intermediate ◊,[A] + [B] → [◊] → [Products] + light
 W
W4,4′-Diamino-2,2′-stilbenedisulfonic acid is the organic compound with the formula (H2NC6H3SO3H)2C2H2. It is a white, water-soluble solid. Structurally, it is a derivative of trans-stilbene, containing amino and sulfonic acid functional groups on each of the two phenyl rings.
 W
WeFluor nanocrystals are a class of fluorophores made of semiconductor quantum dots. The nanocrystals can be provided as either primary amine, carboxylate, or non-functional groups on the surface, allowing conjugation to biomolecules of a researcher's choice. The nanocrystals can be conjugated to primary antibodies which are used for flow cytometry, immunohistochemistry, microarrays, in vivo imaging and microscopy.
 W
WElectrochemiluminescence or electrogenerated chemiluminescence (ECL) is a kind of luminescence produced during electrochemical reactions in solutions. In electrogenerated chemiluminescence, electrochemically generated intermediates undergo a highly exergonic reaction to produce an electronically excited state that then emits light upon relaxation to a lower-level state. This wavelength of the emitted photon of light corresponds to the energy gap between these two states. ECL excitation can be caused by energetic electron transfer (redox) reactions of electrogenerated species. Such luminescence excitation is a form of chemiluminescence where one/all reactants are produced electrochemically on the electrodes.
 W
WElectroluminescence (EL) is an optical phenomenon and electrical phenomenon in which a material emits light in response to the passage of an electric current or to a strong electric field. This is distinct from black body light emission resulting from heat (incandescence), a chemical reaction (chemiluminescence), sound (sonoluminescence), or other mechanical action (mechanoluminescence).
 W
WElectroluminescent Displays (ELDs) are a type of Flat panel display created by sandwiching a layer of electroluminescent material such as GaAs between two layers of conductors. When current flows, the layer of material emits radiation in the form of visible light. Electroluminescence (EL) is an optical and electrical phenomenon where a material emits light in response to an electric current passed through it, or to a strong electric field. The term "electroluminescent display" describes displays that use neither LED nor OLED devices, that instead use traditional electroluminescent materials. Beneq is the only manufacturer of TFEL and TAESL displays. The structure of a TFEL is similar to that of a passive matrix LCD or OLED display, and TAESL displays are essentially transparent TEFL displays with transparent electrodes. TAESL displays can have a transparency of 80%. Both TEFL and TAESL displays use chip-on-glass technology, which mounts the display driver IC directly on one of the edges of the display. TAESL displays can be embedded onto glass sheets. Unlike LCDs, TFELs are much more rugged and can operate at temperatures from -60 to 105°C and unlike OLEDs, TFELs can operate for 100,000 hours without considerable burn-in, only losing about 80% of its initial brightness. The electroluminescent material is deposited using atomic layer deposition, which is a process that deposits one 1-atom thick layer at a time.
 W
WElectroluminescent wire is a thin copper wire coated in a phosphor that produces light through electroluminescence when an alternating current is applied to it. It can be used in a wide variety of applications—vehicle and structure decoration, safety and emergency lighting, toys, clothing etc.—much as rope light or Christmas lights are often used. Unlike these types of strand lights, EL wire is not a series of points, but produces a 360 degree unbroken line of visible light. Its thin diameter makes it flexible and ideal for use in a variety of applications such as clothing or costumes.
 W
WA fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.
 W
WA formation light is a type of thin film electroluminescent light that assists aircraft flying in formation in low visibility environments.
 W
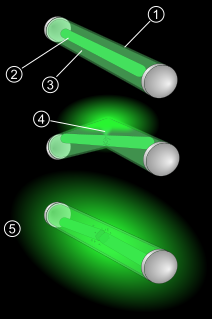
WA glow stick is a self-contained, short-term light-source. It consists of a translucent plastic tube containing isolated substances that, when combined, make light through chemiluminescence, so it does not require an external energy source. The light cannot be turned off and can be used only once. Glow sticks are often used for recreation, but may also be relied upon for light during military, police, fire, or emergency medical services operations. They are also used by military and police to mark "clear" areas.
 W
WIncandescence is the emission of electromagnetic radiation from a hot body as a result of its high temperature. The term derives from the Latin verb incandescere, to glow white.
 W
WIndiglo is a product feature on watches marketed by Timex, incorporating an electroluminescent panel as a backlight for even illumination of the watch dial.
 W
WKasha's rule is a principle in the photochemistry of electronically excited molecules. The rule states that photon emission occurs in appreciable yield only from the lowest excited state of a given multiplicity. It is named for American spectroscopist Michael Kasha, who proposed it in 1950.
 W
WLume is a short term for the luminous phosphorescent glowing solution applied on watch dials. There are some people who "relume" watches, or replace faded lume. Formerly, lume consisted mostly of radium; however, radium is radioactive and has been mostly replaced on new watches by less bright, but less toxic compounds.
 W
WLuminous paint or luminescent paint is paint that exhibits luminescence. In other words, it gives off visible light through fluorescence, phosphorescence, or radioluminescence. There are three types of luminous paints.
 W
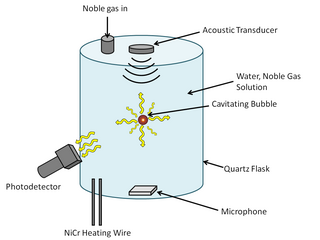
WSonoluminescence is a phenomenon that occurs when a small gas bubble is acoustically suspended and periodically driven in a liquid solution at ultrasonic frequencies, resulting in bubble collapse, cavitation, and light emission. The thermal energy that is released from the bubble collapse is so great that it can cause weak light emission. The mechanism of the light emission remains uncertain, but some of the current theories, which are categorized under either thermal or electrical processes, are Bremsstrahlung radiation, argon rectification hypothesis, and hot spot. Some researchers are beginning to favor thermal process explanations as temperature differences have consistently been observed with different methods of spectral analysis. In order to understand the light emission mechanism, it is important to know what is happening in the bubble's interior and at the bubble's surface.
 W
WOptical brighteners, optical brightening agents (OBAs), fluorescent brightening agents (FBAs), or fluorescent whitening agents (FWAs), are chemical compounds that absorb light in the ultraviolet and violet region of the electromagnetic spectrum, and re-emit light in the blue region by fluorescence. These additives are often used to enhance the appearance of color of fabric and paper, causing a "whitening" effect; they make intrinsically yellow/orange materials look less so, by compensating the deficit in blue and purple light reflected by the material, with the blue and purple optical emission of the fluorophore.
 W
WPeroxyoxalates are esters initially formed by the reaction of hydrogen peroxide with oxalate diesters or oxalyl chloride, with or without base, although the reaction is much faster with base:
 W
WA phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or visible light, and cathodoluminescent substances which glow when struck by an electron beam in a cathode ray tube.
 W
WPhosphorescence is a type of photoluminescence related to fluorescence. Unlike fluorescence, a phosphorescent material does not immediately re-emit the radiation it absorbs. The slower time scales of the re-emission are associated with "forbidden" energy state transitions in quantum mechanics. As these transitions occur very slowly in certain materials, absorbed radiation is re-emitted at a lower intensity for up to several hours after the original excitation.
 W
WA phosphoroscope is piece of experimental equipment devised in 1857 by physicist A. E. Becquerel to measure how long it takes a phosphorescent material to stop glowing after it has been excited.
 W
WPhotoluminescence is light emission from any form of matter after the absorption of photons. It is one of many forms of luminescence and is initiated by photoexcitation, hence the prefix photo-. Following excitation various relaxation processes typically occur in which other photons are re-radiated. Time periods between absorption and emission may vary: ranging from short femtosecond-regime for emission involving free-carrier plasma in inorganic semiconductors up to milliseconds for Phosphorescence processes in molecular systems; and under special circumstances delay of emission may even span to minutes or hours.
 W
WRadioluminescence is the phenomenon by which light is produced in a material by bombardment with ionizing radiation such as alpha particles, beta particles, or gamma rays. Radioluminescence is used as a low level light source for night illumination of instruments or signage. Radioluminescent paint used to be used for clock hands and instrument dials, enabling them to be read in the dark. Radioluminescence is also sometimes seen around high-power radiation sources, such as nuclear reactors and radioisotopes.
 W
WRadium dials are watch, clock and other instrument dials painted with radioluminescent paint containing radium-226. Radium dial production peaked in the first decade of the 20th century as radiation poisoning was then unknown; subsequently, radium dials have largely been replaced by phosphorescent- or occasionally tritium-based light sources.
 W
WSensor Coating Systems (SCS) is an instrumentation company that develops temperature-sensing technologies. It was formerly part of Southside Thermal Sciences.
 W
WSonoluminescence is the emission of short bursts of light from imploding bubbles in a liquid when excited by sound.
 W
W(E)-Stilbene, commonly known as trans-stilbene, is an organic compound represented by the condensed structural formula C6H5CH=CHC6H5. Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituents on each end of the carbon–carbon double bond. It has an (E) stereochemistry, meaning that the phenyl groups are located on opposite sides of the double bond, the opposite of its geometric isomer, cis-stilbene. Trans-stilbene occurs as a white crystalline solid at room temperature and is highly soluble in organic solvents. It can be converted to cis-stilbene photochemically, and further reacted to produce phenanthrene.
 W
W(Z)-Stilbene is a diarylethene, that is, a hydrocarbon consisting of a cis ethene double bond substituted with a phenyl group on both carbon atoms of the double bond. The name stilbene was derived from the Greek word stilbos, which means shining.
 W
WStrontium aluminate (SRA, SrAl) is an aluminate compound with the chemical formula SrAl2O4 (sometimes written as SrO·Al2O3). It is a pale yellow, monoclinic crystalline powder that is odorless and non-flammable. When activated with a suitable dopant (e.g. europium, written as Eu:SrAl2O4), it acts as a photoluminescent phosphor with long persistence of phosphorescence.
 W
WSuper-LumiNova is a brand name under which strontium aluminate–based non-radioactive and nontoxic photoluminescent or afterglow pigments for illuminating markings on watch dials, hands and bezels, etc. in the dark are marketed. This technology offers up to ten times higher brightness than previous zinc sulfide–based materials.
 W
WTagging of postage stamps means that the stamps are printed on luminescent paper or with luminescent ink to facilitate automated mail processing. Both fluorescence and phosphorescence are used. The same stamp may have been printed with and without these luminescent features, the two varieties are referred to as tagged and untagged, respectively.
 W
WThermoluminescence is a form of luminescence that is exhibited by certain crystalline materials, such as some minerals, when previously absorbed energy from electromagnetic radiation or other ionizing radiation is re-emitted as light upon heating of the material. The phenomenon is distinct from that of black-body radiation.
 W
WThermoluminescence dating (TL) is the determination, by means of measuring the accumulated radiation dose, of the time elapsed since material containing crystalline minerals was either heated or exposed to sunlight (sediments). As a crystalline material is heated during measurements, the process of thermoluminescence starts. Thermoluminescence emits a weak light signal that is proportional to the radiation dose absorbed by the material. It is a type of luminescence dating.
 W
WTriboluminescence is an optical phenomenon in which light is generated when a material is mechanically pulled apart, ripped, scratched, crushed, or rubbed. The phenomenon is not fully understood, but appears to be caused by the separation and reunification of static electrical charges. The term comes from the Greek τρίβειν and the Latin lumen (light). Triboluminescence can be observed when breaking sugar crystals and peeling adhesive tapes.
 W
WUndark was a trade name for luminous paint made with a mixture of radioactive radium and zinc sulfide, as produced by the U.S. Radium Corporation between 1917 and 1938. It was used primarily in watch and clock dials. The people working in the industry who applied the radioactive paint became known as the Radium Girls, because many of them became ill and some died from exposure to the radiation emitted by the radium contained within the product. The product was the direct cause of radium jaw in the dial painters. Undark was also available as a kit for general consumer use and marketed as glow-in-the-dark paint.
 W
WMarcel Joseph Vogel was a research scientist working at the IBM San Jose Research Center for 27 years. He is sometimes referred to as Dr. Vogel, although this title was based on an honorary degree, not a Ph.D. Later in his career, he became interested in various theories of quartz crystals and other occult and esoteric fields of study. The Vogel Crystal type cut was created by him.
 W
WZinc sulfide is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics.