 W
WHydrogen storage is a term used for any of several methods for storing hydrogen for later use. These methods encompass mechanical approaches such as high pressures and low temperatures, or chemical compounds that release H2 upon demand. While large amounts of hydrogen is produced, it is mostly consumed at the site of production, notably for the synthesis of ammonia. For many years hydrogen has been stored as compressed gas or cryogenic liquid, and transported as such in cylinders, tubes, and cryogenic tanks for use in industry or as propellant in space programs. Interest in using hydrogen for on-board storage of energy in zero-emissions vehicles is motivating the development of new methods of storage, more adapted to this new application. The overarching challenge is the very low boiling point of H2: it boils around 20.268 K (−252.882 °C or −423.188 °F). Achieving such low temperatures requires significant energy.
 W
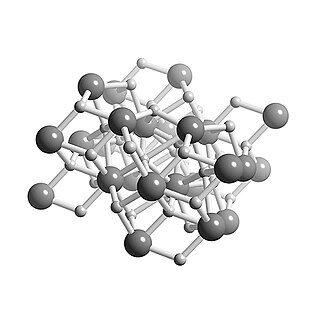
WCalcium hydride is the chemical compound with the formula CaH2, and is therefore an alkaline earth hydride. This grey powder (white if pure, which is rare) reacts vigorously with water liberating hydrogen gas. CaH2 is thus used as a drying agent, i.e. a desiccant.
 W
WA cascade filling system is a high pressure gas cylinder storage system which is used for the refilling of smaller compressed gas cylinders. In some applications, each of the large cylinders is filled by a compressor, otherwise they may be filled remotely and replaced when the pressure is too low for effective transfer. The cascade system allows small cylinders to be filled without a compressor. In addition, a cascade system is useful as a reservoir to allow a low-capacity compressor to meet the demand of filling several small cylinders in close succession, with longer intermediate periods during which the storage cylinders can be recharged.
 W
WCompressed hydrogen (CH2, CGH2 or CGH2) is the gaseous state of the element hydrogen kept under pressure. Compressed hydrogen in hydrogen tanks at 350 bar (5,000 psi) and 700 bar (10,000 psi) is used for mobile hydrogen storage in hydrogen vehicles. It is used as a fuel gas.
 W
WHydrogen tube trailers are semi-trailers that consist of 4 to 36 cluster high-pressure hydrogen tanks varying in length from 20 feet (6.10 m) for small tubes to 53 feet (16.15 m) on jumbo tube trailers. They are part of the hydrogen highway and usually precede a local hydrogen station.
 W
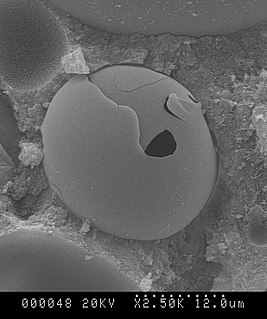
WGlass microspheres are microscopic spheres of glass manufactured for a wide variety of uses in research, medicine, consumer goods and various industries. Glass microspheres are usually between 1 and 1000 micrometers in diameter, although the sizes can range from 100 nanometers to 5 millimeters in diameter. Hollow glass microspheres, sometimes termed microballoons or glass bubbles, have diameters ranging from 10 to 300 micrometers.
 W
WA Hydrogen tank is used for hydrogen storage. The first type IV hydrogen tanks for compressed hydrogen at 700 bars were demonstrated in 2001, the first fuel cell vehicles on the road with type IV tanks are the Toyota FCHV, Mercedes-Benz F-Cell and the GM HydroGen4.
 W
WLiquid hydrogen (LH2 or LH2) is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.
 W
WSodium aluminium hydride or sodium alanate is an inorganic compound with the chemical formula NaAlH4. It is a white pyrophoric solid that dissolves in tetrahydrofuran (THF), but not in diethyl ether or hydrocarbons. It has been evaluated as an agent for the reversible storage of hydrogen and it is used as a reagent for the chemical synthesis of organic compounds. Similar to lithium aluminium hydride, it is a salt consisting of separated sodium cations and tetrahedral AlH−4 anions.