 W
WCis–trans isomerism, also known as geometric isomerism or configurational isomerism, is a term used in organic chemistry. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, cis indicates that the functional groups are on the same side of the carbon chain while trans conveys that functional groups are on opposing sides of the carbon chain. Cis-trans isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are rotated into a different orientation in three-dimensional space. It is not to be confused with E–Z isomerism, which is an absolute stereochemical description. In general, stereoisomers contain double bonds that do not rotate, or they may contain ring structures, where the rotation of bonds is restricted or prevented. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes. Cis and trans descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "syn" and "anti" are used.
 W
WClaus' benzene (C6H6) is a hypothetical hydrocarbon and an isomer of benzene. It was proposed by Adolf Karl Ludwig Claus in 1867 as a possible structure for benzene at a time when the structure of benzene was still being debated. The molecule can be described as a hexagon with carbon atoms positioned at the corners, with each carbon connected to its two ortho carbons (the nearest carbons) and the one para carbon connected diametrically. High strain energy makes its synthesis impossible. Although it is often referred to alongside Dewar benzene and Prismane, it is not possible to synthesise it, which Dewar benzene and Prismane can be.
 W
WIn chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds. While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations, conformations that correspond to local minima on the energy surface are specifically called conformational isomers or conformers. Conformations that correspond to local maxima on the energy surface are the transition states between the local-minimum conformational isomers. Rotations about single bonds involve overcoming a rotational energy barrier to interconvert one conformer to another. If the energy barrier is low, there is free rotation and a sample of the compound exists as a rapidly equilibrating mixture of multiple conformers; if the energy barrier is high enough then there is restricted rotation, a molecule may exist for a relatively long time period as a stable rotational isomer or rotamer. When the time scale for interconversion is long enough for isolation of individual rotamers, the isomers are termed atropisomers. The ring-flip of substituted cyclohexanes constitutes another common form of conformational isomerism.
 W
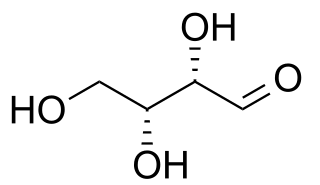
WDiastereomers are a type of a stereoisomer. Diastereomers are defined as non-mirror image non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two.
 W
WIn chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable, much as one's left and right hands are mirror images of each other that cannot appear identical simply by reorientation. A single chiral atom or similar structural feature in a compound causes that compound to have two possible structures which are non-superposable, each a mirror image of the other. Each member of the pair is termed an enantiomorph ; the structural property is termed enantiomerism. The presence of multiple chiral features in a given compound increases the number of geometric forms possible, though there may still be some perfect-mirror-image pairs.
 W
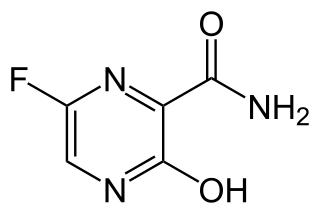
WFavipiravir, sold under the brand name Avigan among others, is an antiviral medication used to treat influenza in Japan. It is also being studied to treat a number of other viral infections. Like the experimental antiviral drugs T-1105 and T-1106, it is a pyrazinecarboxamide derivative.
 W
WIn chemistry, isomers are molecules or polyatomic ions with identical molecular formulas — that is, same number of atoms of each element — but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
 W
WIn stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.
 W
WTautomers are structural isomers of chemical compounds that readily interconvert. This reaction commonly results in the relocation of a proton. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life.