 W
WBoron nitride nanosheet is a two-dimensional crystalline form of the hexagonal boron nitride (h-BN), which has a thickness of one to few atomic layers. It is similar in geometry to its all-carbon analog graphene, but has very different chemical and electronic properties – contrary to the black and highly conducting graphene, BN nanosheets are electrical insulators with a band gap of ~5.9 eV, and therefore appear white in color.
 W
WGermanene is a material made up of a single layer of germanium atoms. The material is created in a process similar to that of silicene and graphene, in which high vacuum and high temperature are used to deposit a layer of germanium atoms on a substrate. High-quality thin films of germanene have revealed unusual two-dimensional structures with novel electronic properties suitable for semiconductor device applications and materials science research.
 W
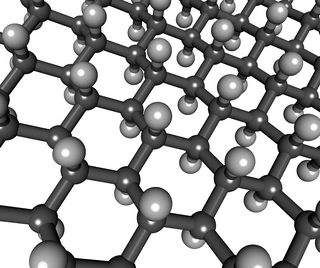
WGraphane is a two-dimensional polymer of carbon and hydrogen with the formula unit (CH)n where n is large. Partial hydrogenation is then hydrogenated graphene.
 W
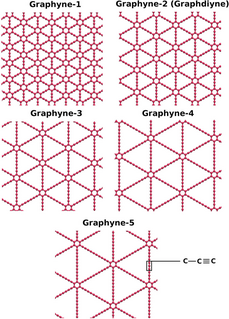
WGraphyne is an allotrope of carbon. Its structure is one-atom-thick planar sheets of sp and sp2-bonded carbon atoms arranged in crystal lattice. It can be seen as a lattice of benzene rings connected by acetylene bonds. Depending on the content of acetylene groups, graphyne can be considered a mixed hybridization, spn, where 1 < n < 2, and thus differs from the hybridization of graphene and diamond.
 W
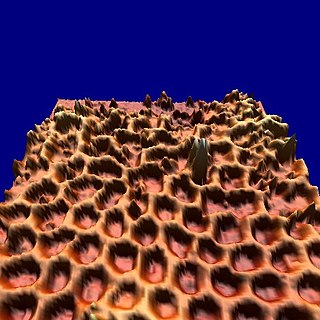
WThe nanomesh is an inorganic nanostructured two-dimensional material, similar to graphene. It was discovered in 2003 at the University of Zurich, Switzerland.
 W
WPenta-silicene or Pentasilicene denotes a silicon-based two-dimensional (2D) structure, a cousin of silicene, composed entirely of Si pentagons, in analogy with Penta-graphene,a hypothetical variant of graphene. As of 2017 such a structure has only been obtained synthetically as one-dimensional nanoribbons (1D-NRs) grown on a silver (110) substrate. These nanoribbons adopt a highly ordered chiral arrangement in single- and/or double-strands. They were discovered in 2005 upon depositing Si onto the Ag(110) surface held at room temperature or at about 200 °C, and observed in Scanning Tunneling Microscopy,. However, their unique atomic structure was unveiled only in 2016 through thorough Density Functional Theory calculations and simulations of the STM images. It consists of alternating Si pentagons residing along a missing row formed at the silver surface during the growth process. In the Penta-silicene NRs each Si pentagonal moiety displays an envelope conformation whereby four atoms are coplanar and a fifth flap atom protrudes out of the surface. The pentagons, nevertheless, do not deviate much from regular ones. DNRs consist of two SNRs with the same handedness running in parallel along two missing rows separated by two Ag lattice constants.
 W
WIn nanobiotechnology, a peptoid nanosheet is a synthetic protein structure made from peptoids. Peptoid nanosheets have a thickness of about three nanometers and a length of up to 100 micrometers, meaning that they have a two-dimensional, flat shape that resembles paper on the nanoscale.
 W
WSilicene is a two-dimensional allotrope of silicon, with a hexagonal honeycomb structure similar to that of graphene. Contrary to graphene, silicene is not flat, but has a periodically buckled topology; the coupling between layers in silicene is much stronger than in multilayered graphene; and the oxidized form of silicene, 2D silica, has a very different chemical structure from graphene oxide.
 W
WStanene is a single layer or 2D material and a 2D topological insulator. It is composed of tin atoms arranged in a single, hexagonal layer, in a manner similar to graphene. Its name combines stannum with the suffix -ene used by graphene.
 W
WTitanate (IV) nanosheets (TiNSs) have a 2D structure where TiO6 octahedra are edge-linked in a lepidocrocite-type 2D lattice with chemical formula HxTi2—x/4☐x/4O4 ⦁ H2O (x~0.7; ☐, vacancy). Titanate nanosheets may be regarded as sheets with molecular thickness and infinite planar dimensions. TiNSs are typically formed via liquid-phase exfoliation of protonic titanate. In inorganic layered materials, individual layers are bound to each other by van der Waals interactions if they are neutral, and additional Coulomb interactions if they are composed of oppositely charged layers. Through liquid-phase exfoliation, these individual sheets of layered materials can be efficiently separated using an appropriate solvent, creating single-layer colloidal suspensions. Solvents must have an interaction energy with the layers that is greater than the interaction energy between two layers. In situ X-Ray diffraction data indicates that TiNSs can be treated as macromolecules with a sufficient amount of solvent in between layers so that they behave as individual sheets.
 W
WTwo-dimensional silica (2D silica) is a layered polymorph of silicon dioxide. Two varieties of 2D silica, both of hexagonal crystal symmetry, have been grown so far on various metal substrates. One is based on SiO4 tetrahedra, which are covalently bonded to the substrate. The second comprises graphene-like fully saturated sheets, which interact with the substrate via weak van der Waals bonds. One sheet of the second 2D silica variety is also called hexagonal bilayer silica (HBS); it can have either ordered or disordered (amorphous) structure.