 W
WMedicinal chemistry and pharmaceutical chemistry are disciplines at the intersection of chemistry, especially synthetic organic chemistry, and pharmacology and various other biological specialties, where they are involved with design, chemical synthesis and development for market of pharmaceutical agents, or bio-active molecules (drugs).
 W
WADME is an abbreviation in pharmacokinetics and pharmacology for "absorption, distribution, metabolism, and excretion", and describes the disposition of a pharmaceutical compound within an organism. The four criteria all influence the drug levels and kinetics of drug exposure to the tissues and hence influence the performance and pharmacological activity of the compound as a drug. Sometimes, liberation and/or toxicity are also considered, yielding LADME, ADMET, or LADMET.
 W
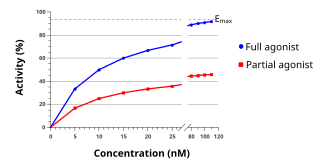
WAn agonist is a chemical that binds to a receptor and activates the receptor to produce a biological response. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist.
 W
WBindingDB is a public, web-accessible database of measured binding affinities, focusing chiefly on the interactions of proteins considered to be candidate drug-targets with ligands that are small, drug-like molecules. As of March, 2011, BindingDB contains about 650,000 binding data, for 5,700 protein targets and 280,000 small molecules. BindingDB also includes a small collection of host–guest binding data of interest to chemists studying supramolecular systems.
 W
WJeannette Elizabeth Brown is a retired American organic medicinal chemist, historian, and author.
 W
WThe Craig plot, named after Paul N. Craig, is a plot of two substituent parameters used in rational drug design.
 W
WIn the field of molecular modeling, docking is a method which predicts the preferred orientation of one molecule to a second when bound to each other to form a stable complex. Knowledge of the preferred orientation in turn may be used to predict the strength of association or binding affinity between two molecules using, for example, scoring functions.
 W
WDrug design, often referred to as rational drug design or simply rational design, is the inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic small molecule that activates or inhibits the function of a biomolecule such as a protein, which in turn results in a therapeutic benefit to the patient. In the most basic sense, drug design involves the design of molecules that are complementary in shape and charge to the biomolecular target with which they interact and therefore will bind to it. Drug design frequently but not necessarily relies on computer modeling techniques. This type of modeling is sometimes referred to as computer-aided drug design. Finally, drug design that relies on the knowledge of the three-dimensional structure of the biomolecular target is known as structure-based drug design. In addition to small molecules, biopharmaceuticals including peptides and especially therapeutic antibodies are an increasingly important class of drugs and computational methods for improving the affinity, selectivity, and stability of these protein-based therapeutics have also been developed.
 W
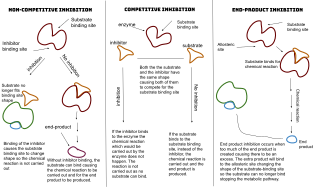
WAn enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. By binding to enzymes' active sites, inhibitors reduce the compatibility of substrate and enzyme and this leads to the inhibition of Enzyme-Substrate complexes' formation, preventing the catalysis of reactions and decreasing the amount of product produced by a reaction. It can be said that as the concentration of enzyme inhibitors increases, the rate of enzyme activity decreases, and thus, the amount of product produced is inversely proportional to the concentration of inhibitor molecules. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.
 W
WExtrafarma is the drugstore chain owned by Ultrapar. The company is among the top 10 largest pharmacy chains in Brazil, with stores located throughout the north, northeast and southern regions of the country.
 W
WThe first pass effect is a phenomenon of drug metabolism whereby the concentration of a drug, specifically when administered orally, is greatly reduced before it reaches the systemic circulation. It is the fraction of drug lost during the process of absorption which is generally related to the liver and gut wall. Notable drugs that experience a significant first-pass effect are imipramine, morphine, propranolol, buprenorphine, diazepam, midazolam, pethidine, tetrahydrocannabinol (THC), ethanol, cimetidine, lidocaine, and nitroglycerin (GTN). In contrast some drugs are enhanced in potency: for example, the effect of the most commonly considered active ingredient in cannabis, THC, is enhanced by transformation of a significant portion into 11-hydroxy-THC that more readily crosses the blood-brain barrier and thus achieves greater potency than the original THC.
 W
WImmunomodulatory imide drugs (IMiDs) are a class of immunomodulatory drugs containing an imide group. The IMiD class includes thalidomide and its analogues. These drugs may also be referred to as 'Cereblon modulators'. Cereblon is the protein targeted by this class of drugs.
 W
WLipinski's rule of five, also known as Pfizer's rule of five or simply the rule of five (RO5), is a rule of thumb to evaluate druglikeness or determine if a chemical compound with a certain pharmacological or biological activity has chemical properties and physical properties that would make it a likely orally active drug in humans. The rule was formulated by Christopher A. Lipinski in 1997, based on the observation that most orally administered drugs are relatively small and moderately lipophilic molecules.
 W
WLipophilic efficiency (LiPE), sometimes referred to as ligand-lipophilicity efficiency (LLE) is a parameter used in drug design and drug discovery to evaluate the quality of research compounds, linking potency and lipophilicity in an attempt to estimate druglikeness. For a given compound LiPE is defined as the pIC50 (or pEC50) of interest minus the LogP of the compound.
 W
WIn pharmacology, the term mechanism of action (MOA) refers to the specific biochemical interaction through which a drug substance produces its pharmacological effect. A mechanism of action usually includes mention of the specific molecular targets to which the drug binds, such as an enzyme or receptor. Receptor sites have specific affinities for drugs based on the chemical structure of the drug, as well as the specific action that occurs there.
 W
WPharmacodynamics (PD) is the study of the biochemical and physiologic effects of drugs. The effects can include those manifested within animals, microorganisms, or combinations of organisms.
 W
WA pharmacophore is an abstract description of molecular features that are necessary for molecular recognition of a ligand by a biological macromolecule. IUPAC defines a pharmacophore to be "an ensemble of steric and electronic features that is necessary to ensure the optimal supramolecular interactions with a specific biological target and to trigger its biological response". A pharmacophore model explains how structurally diverse ligands can bind to a common receptor site. Furthermore, pharmacophore models can be used to identify through de novo design or virtual screening novel ligands that will bind to the same receptor.
 W
WPharmacy is the clinical health science that links medical science with chemistry and it is charged with the discovery, production, disposal, safe and effective use, and control of medications and drugs. The practice of pharmacy requires excellent knowledge of drugs, their mechanism of action, side effects, interactions, mobility and toxicity. At the same time, it requires knowledge of treatment and understanding of the pathological process. Some specialties of pharmacists, such as that of clinical pharmacists, require other skills, e.g. knowledge about the acquisition and evaluation of physical and laboratory data.
 W
WThe polar surface area (PSA) or topological polar surface area (TPSA) of a molecule is defined as the surface sum over all polar atoms or molecules, primarily oxygen and nitrogen, also including their attached hydrogen atoms.
 W
WRadiopharmaceuticals, or medicinal radiocompounds, are a group of pharmaceutical drugs containing radioactive isotopes. Radiopharmaceuticals can be used as diagnostic and therapeutic agents. Radiopharmaceuticals emit radiation themselves, which is different from contrast media which absorb or alter external electromagnetism or ultrasound. Radiopharmacology is the branch of pharmacology that specializes in these agents.
 W
WRadiopharmacology is radiochemistry applied to medicine and thus the pharmacology of radiopharmaceuticals. Radiopharmaceuticals are used in the field of nuclear medicine as radioactive tracers in medical imaging and in therapy for many diseases. Many radiopharmaceuticals use technetium-99m (Tc-99m) which has many useful properties as a gamma-emitting tracer nuclide. In the book Technetium a total of 31 different radiopharmaceuticals based on Tc-99m are listed for imaging and functional studies of the brain, myocardium, thyroid, lungs, liver, gallbladder, kidneys, skeleton, blood and tumors.
 W
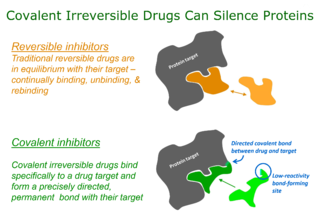
WTargeted covalent inhibitors (TCIs) or Targeted covalent drugs are rationally designed inhibitors that bind and then bond to their target proteins. These inhibitors possess a bond-forming functional group of low chemical reactivity that, following binding to the target protein, is positioned to react rapidly with a proximate nucleophilic residue at the target site to form a bond.
 W
WA therapy or medical treatment is the attempted remediation of a health problem, usually following a medical diagnosis.
 W
WAnabella P. Villalobos is a medicinal chemist and senior pharmaceutical executive at Biogen.
 W
WIn organic chemistry, a vinyl sulfone is a functional group consisting of a vinyl group bonded to a sulfone group. Specific compounds containing this functional group are divinyl sulfone, phenyl vinyl sulfone, methyl vinyl sulfone, and ethyl vinyl sulfone.
 W
W W
W W
W W
W W
W W
W W
W W
W W
W W
W