 W
WIn the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered.
 W
WThe 5-HT3 antagonists, informally known as "setrons", are a class of drugs that act as receptor antagonists at the 5-HT3 receptor, a subtype of serotonin receptor found in terminals of the vagus nerve and in certain areas of the brain. With the notable exceptions of alosetron and cilansetron, which are used in the treatment of irritable bowel syndrome, all 5-HT3 antagonists are antiemetics, used in the prevention and treatment of nausea and vomiting. They are particularly effective in controlling the nausea and vomiting produced by cancer chemotherapy and are considered the gold standard for this purpose.
 W
WAnabaseine (3,4,5,6-Tetrahydro-2,3’-bipyridine) is an alkaloid toxin produced by Nemertines and Aphaenogaster ants. It is structurally similar to nicotine and anabasine. Similarly, it has been shown to act as an agonist on most nicotinic acetylcholine receptors in the central nervous system and peripheral nervous system.
 W
Wβ adrenergic receptor antagonists were initially developed in the 1960s, for the treatment of angina pectoris but are now also used for hypertension, congestive heart failure and certain arrhythmias. In the 1950s, dichloroisoproterenol (DCI) was discovered to be a β-antagonist that blocked the effects of sympathomimetic amines on bronchodilation, uterine relaxation and heart stimulation. Although DCI had no clinical utility, a change in the compound did provide a clinical candidate, pronethalol, which was introduced in 1962.
 W
WChemical similarity refers to the similarity of chemical elements, molecules or chemical compounds with respect to either structural or functional qualities, i.e. the effect that the chemical compound has on reaction partners in inorganic or biological settings. Biological effects and thus also similarity of effects are usually quantified using the biological activity of a compound. In general terms, function can be related to the chemical activity of compounds.
 W
WChemogenomics, or chemical genomics, is the systematic screening of targeted chemical libraries of small molecules against individual drug target families with the ultimate goal of identification of novel drugs and drug targets. Typically some members of a target library have been well characterized where both the function has been determined and compounds that modulate the function of those targets have been identified. Other members of the target family may have unknown function with no known ligands and hence are classified as orphan receptors. By identifying screening hits that modulate the activity of the less well characterized members of the target family, the function of these novel targets can be elucidated. Furthermore, the hits for these targets can be used as a starting point for drug discovery. The completion of the human genome project has provided an abundance of potential targets for therapeutic intervention. Chemogenomics strives to study the intersection of all possible drugs on all of these potential targets.
 W
WIn the field of drug discovery, classical pharmacology, also known as forward pharmacology, or phenotypic drug discovery (PDD), relies on phenotypic screening of chemical libraries of synthetic small molecules, natural products or extracts to identify substances that have a desirable therapeutic effect. Using the techniques of medicinal chemistry, the potency, selectivity, and other properties of these screening hits are optimized to produce candidate drugs.
 W
WClinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.
 W
WCOVID‑19 drug development is the research process to develop preventative therapeutic prescription drugs that would alleviate the severity of coronavirus disease 2019 (COVID‑19). Internationally by November 2020, several hundred drug companies, biotechnology firms, university research groups, and health organizations were developing over 500 potential therapies for COVID‑19 disease in various stages of preclinical or clinical research.
 W
WBisphosphonates are an important class of drugs originally introduced about half a century ago. They are used for the treatment of osteoporosis and other bone disorders that cause bone fragility and diseases where bone resorption is excessive. Osteoporosis is common in post-menopausal women and patients in corticosteroid treatment where biphosphonates have been proven a valuable treatment and also used successfully against Paget's disease, myeloma, bone metastases and hypercalcemia. Bisphosphonates reduce breakdown of bones by inhibiting osteoclasts, they have a long history of use and today there are a few different types of bisphosphonate drugs on the market around the world.
 W
WIn the field of molecular modeling, docking is a method which predicts the preferred orientation of one molecule to a second when bound to each other to form a stable complex. Knowledge of the preferred orientation in turn may be used to predict the strength of association or binding affinity between two molecules using, for example, scoring functions.
 W
WDrug design, often referred to as rational drug design or simply rational design, is the inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic small molecule that activates or inhibits the function of a biomolecule such as a protein, which in turn results in a therapeutic benefit to the patient. In the most basic sense, drug design involves the design of molecules that are complementary in shape and charge to the biomolecular target with which they interact and therefore will bind to it. Drug design frequently but not necessarily relies on computer modeling techniques. This type of modeling is sometimes referred to as computer-aided drug design. Finally, drug design that relies on the knowledge of the three-dimensional structure of the biomolecular target is known as structure-based drug design. In addition to small molecules, biopharmaceuticals including peptides and especially therapeutic antibodies are an increasingly important class of drugs and computational methods for improving the affinity, selectivity, and stability of these protein-based therapeutics have also been developed.
 W
WDrug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug.
 W
WDynamic combinatorial chemistry (DCC); also known as constitutional dynamic chemistry (CDC) is a method to the generation of new molecules formed by reversible reaction of simple building blocks under thermodynamic control. The library of these reversibly interconverting building blocks is called a dynamic combinatorial library (DCL). All constituents in a DCL are in equilibrium, and their distribution is determined by their thermodynamic stability within the DCL. The interconversion of these building blocks may involve covalent or non-covalent interactions. When a DCL is exposed to an external influence, the equilibrium shifts and those components that interact with the external influence are stabilised and amplified, allowing more of the active compound to be formed.
 W
WIn pharmacology, GABAA receptor positive allosteric modulators are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.
 W
WLipase inhibitors belong to a drug class that is used as an antiobesity agent. Their mode of action is to inhibit gastric and pancreatic lipases, enzymes that play an important role in the digestion of dietary fat. Lipase inhibitors are classified in the ATC-classification system as A08AB . Numerous compounds have been either isolated from nature, semi-synthesized, or fully synthesized and then screened for their lipase inhibitory activity but the only lipase inhibitor on the market is orlistat . Lipase inhibitors have also shown anticancer activity, by inhibiting fatty acid synthase.
 W
WHigh-throughput screening (HTS) is a method for scientific experimentation especially used in drug discovery and relevant to the fields of biology and chemistry. Using robotics, data processing/control software, liquid handling devices, and sensitive detectors, high-throughput screening allows a researcher to quickly conduct millions of chemical, genetic, or pharmacological tests. Through this process one can rapidly identify active compounds, antibodies, or genes that modulate a particular biomolecular pathway. The results of these experiments provide starting points for drug design and for understanding the noninteraction or role of a particular location.
 W
WAttempts at producing a state of general anesthesia can be traced throughout recorded history in the writings of the ancient Sumerians, Babylonians, Assyrians, Egyptians, Indians, and Chinese. During the Middle Ages, which correspond roughly to what is sometimes referred to as the Islamic Golden Age, scientists and other scholars made significant advances in science and medicine in the Muslim world and Eastern world.
 W
WThe history of neuraxial anesthesia goes back to 1885.
 W
WThe history of pharmacy as an independent science dates back to the first third of the 19th century. Before then, pharmacy evolved from antiquity as part of medicine. The history of pharmacy coincides well with the history of medicine, but it's important that there is a distinction between the two topics. Pharmaceuticals is one of the most-researched fields in the academic industry, but the history surrounding that particular topic is sparse compared to the impact its made world-wide. Before the advent of pharmacists, there existed apothecaries that worked alongside priests and physicians in regard to patient care.
 W
WImmunomodulatory imide drugs (IMiDs) are a class of immunomodulatory drugs containing an imide group. The IMiD class includes thalidomide and its analogues. These drugs may also be referred to as 'Cereblon modulators'. Cereblon is the protein targeted by this class of drugs.
 W
WLipinski's rule of five, also known as Pfizer's rule of five or simply the rule of five (RO5), is a rule of thumb to evaluate druglikeness or determine if a chemical compound with a certain pharmacological or biological activity has chemical properties and physical properties that would make it a likely orally active drug in humans. The rule was formulated by Christopher A. Lipinski in 1997, based on the observation that most orally administered drugs are relatively small and moderately lipophilic molecules.
 W
WLipophilic efficiency (LiPE), sometimes referred to as ligand-lipophilicity efficiency (LLE) is a parameter used in drug design and drug discovery to evaluate the quality of research compounds, linking potency and lipophilicity in an attempt to estimate druglikeness. For a given compound LiPE is defined as the pIC50 (or pEC50) of interest minus the LogP of the compound.
 W
WMelatonin receptor agonists are analogues of melatonin that bind to and activate the melatonin receptor. Agonists of the melatonin receptor have a number of therapeutic applications including treatment of sleep disorders and depression. The discovery and development of melatonin receptor agonists was motivated by the need for more potent analogues than melatonin, with better pharmacokinetics and longer half-life. Melatonin receptor agonists were developed with the melatonin structure as a model.
 W
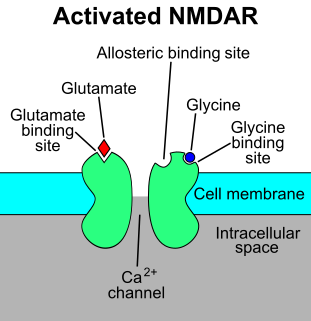
WThe N-methyl-D-aspartate receptor, is a glutamate receptor and ion channel protein found in nerve cells. The NMDA receptor is one of three types of ionotropic glutamate receptors. The other receptors are the AMPA and kainate receptors. It is activated when glutamate and glycine bind to it, and when activated it allows positively charged ions to flow through the cell membrane. The NMDA receptor is very important for controlling synaptic plasticity and memory function.
 W
WmTOR inhibitors are a class of drugs that inhibit the mammalian target of rapamycin (mTOR), which is a serine/threonine-specific protein kinase that belongs to the family of phosphatidylinositol-3 kinase (PI3K) related kinases (PIKKs). mTOR regulates cellular metabolism, growth, and proliferation by forming and signaling through two protein complexes, mTORC1 and mTORC2. The most established mTOR inhibitors are so-called rapalogs, which have shown tumor responses in clinical trials against various tumor types.
 W
WNonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery technology. However, their mechanism of action is complex and not fully understood. NS5A inhibitors were the focus of much attention when they emerged as a part of the first curative treatment for hepatitis C virus (HCV) infections in 2014. Favorable characteristics have been introduced through varied structural changes, and structural similarities between NS5A inhibitors that are clinically approved are readily apparent. Despite the recent introduction of numerous new antiviral drugs, resistance is still a concern and these inhibitors are therefore always used in combination with other drugs.
 W
WIn medicine, the number needed to harm (NNH) is an epidemiological measure that indicates how many persons on average need to be exposed to a risk factor over a specific period to cause harm in an average of one person who would not otherwise have been harmed. It is defined as the inverse of the absolute risk increase, and computed as , where is the incidence in the treated (exposed) group, and is the incidence in the control (unexposed) group. Intuitively, the lower the number needed to harm, the worse the risk factor, with 1 meaning that every exposed person is harmed.
 W
WThe number needed to treat (NNT) is an epidemiological measure used in communicating the effectiveness of a health-care intervention, typically a treatment with medication. The NNT is the average number of patients who need to be treated to prevent one additional bad outcome. It is defined as the inverse of the absolute risk reduction, and computed as , where is the incidence in the treated (exposed) group, and is the incidence in the control (unexposed) group.
 W
WUtako Okamoto was a Japanese medical doctor working as a medical scientist who discovered tranexamic acid in the 1950s in her quest to find a drug that would treat bleeding after childbirth. After publishing results in 1962 she became a chair at Kobe Gakuin University, where she worked from 1966 until her retirement in 1990. Okamoto's career was hampered by a very male dominated environment. During her lifetime she was unable to persuade obstetricians at Kobe to trial the antifibrinolytic agent, which had become a drug on the WHO list of essential medicines in 2009. She lived to see the 2010 beginning of the study of tranexamic acid in 20 000 women with post-partum haemorrhage, but died before its completion in 2016 and the publication of tranexamic acids fatality preventing results in 2017, that she had predicted.
 W
WIn drug development, preclinical development, also named preclinical studies and nonclinical studies, is a stage of research that begins before clinical trials can begin, and during which important feasibility, iterative testing and drug safety data are collected, typically in laboratory animals.
 W
WThe ProTide technology is a prodrug approach used in molecular biology and drug design. It is designed to deliver nucleotide analogues into the cell. It was invented by Professor Chris McGuigan in the early 1990s. They form a critical part of the anti-viral drugs sofosbuvir, tenofovir alafenamide and Remdesivir.
 W
WPsychedelia is an American documentary film from Hard Rain Films, that was initially released in 2015, and then re-released as a revised version in 2020. The film discusses the history of psychedelic drugs and their ability to produce mystical experiences. The therapeutic role of such drugs is considered, and controlled research studies conducted before the 1960s, are discussed. Several study participant users are interviewed. The film was a winner for best documentary film at the New Jersey International Film Festival, and was an official selection at the Southern Utah International Documentary Film Festival (DOCUTAH) and the Orlando Film Festival.
 W
WIn the field of drug discovery, retrometabolic drug design is a strategy for the design of safer drugs either using predictable metabolism to an inactive moiety or using targeted drug delivery approaches. The phrase retrometabolic drug design was coined by Nicholas Bodor. The method is analogous to retrosynthetic analysis where the synthesis of a target molecule is planned backwards. In retrometabolic drug design, metabolic reaction information of drugs is used to design parent drugs whose metabolism and distribution can be controlled to target and eliminate the drug to increase efficacy and minimize undesirable side effects. The new drugs thus designed achieve selective organ and/or therapeutic site drug targeting and produce safe therapeutic agents and safe environmental chemicals. These approaches represent systematic methodologies that thoroughly integrate structure-activity (SAR) and structure-metabolism (SMR) relationships and are aimed at designing safe, locally active compounds with improved therapeutic index.
 W
WIn the field of drug discovery, reverse pharmacology also known as target-based drug discovery (TDD), a hypothesis is first made that modulation of the activity of a specific protein target will have beneficial therapeutic effects. Screening of chemical libraries of small molecules is then used to identify compounds that bind with high affinity to the target. The hits from these screens are then used as starting points for drug discovery. This method became popular after the sequencing of the human genome which allowed rapid cloning and synthesis of large quantities of purified proteins. This method is the most widely used in drug discovery today. Differently than the classical (forward) pharmacology, with the reverse pharmacology approach in vivo efficacy of identified active (lead) compounds is usually performed in the final drug discovery stages.
 W
WSouthern Research is a not-for-profit US 501(c)(3) research organization that conducts basic and applied research for commercial and non-commercial organizations across four divisions: Drug Development, Drug Discovery, Energy & Environment, and Engineering.
 W
WA therapy or medical treatment is the attempted remediation of a health problem, usually following a medical diagnosis.
 W
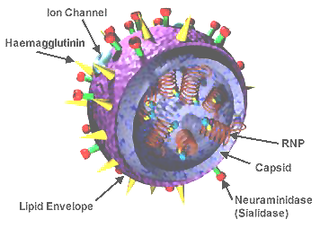
WA universal flu vaccine is a flu vaccine that is effective against all influenza strains regardless of the virus sub type, antigenic drift or antigenic shift. Hence it should not require modification from year to year. As of 2019 there has been no approved universal flu vaccine for general use, but several have been in development.
 W
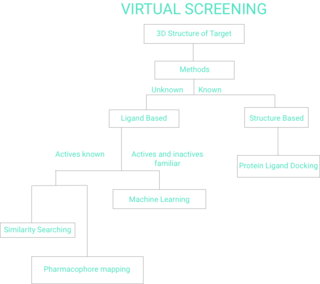
WVirtual screening (VS) is a computational technique used in drug discovery to search libraries of small molecules in order to identify those structures which are most likely to bind to a drug target, typically a protein receptor or enzyme.