 W
WThe history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis of the various branches of chemistry. Examples include the discovery of fire, extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.
 W
WThe Baopuzi, written by the Jin dynasty scholar Ge Hong 葛洪 in 283-343, is divided into esoteric Neipian 內篇 "Inner Chapters" and equally exoteric Waipian 外篇 "Outer Chapters". The Taoist Inner Chapters discuss topics such as techniques to achieve "hsien" 仙, Chinese alchemy, elixirs, and demonology. The Confucian Outer Chapters discuss Chinese literature, Legalism, politics, and society.
 W
WBeevers–Lipson strips were a computational aid for early crystallographers in calculating Fourier transforms to determine the structure of crystals from crystallographic data, enabling the creation of models for complex molecules. They were used from the 1930s until computers with enough power became generally available in the 1960s.
 W
WBowen's Kale was a reference material produced by British chemist Humphry Bowen and used for the calibration of early scientific instruments intended to measure trace elements during the 1960s.
 W
WThe Catalyst Science Discovery Centre is a science and technology museum in Widnes, Halton, North-West England. The centre has interactive exhibits, reconstructed historical scenes, an observatory, a live-science theatre and family workshops. It is next to Spike Island, a public park, located between the River Mersey and the Sankey Canal that has woodlands, wetlands, footpaths and industrial archaeological history.
 W
WThe Chemical History of a Candle was the title of a series of six lectures on the chemistry and physics of flames given by Michael Faraday at the Royal Institution in 1848, as part of the series of Christmas lectures for young people founded by Faraday in 1825 and still given there every year.
 W
WThe chemical revolution, also called the first chemical revolution, was the early modern reformulation of chemistry that culminated in the law of conservation of mass and the oxygen theory of combustion. During the 19th and 20th century, this transformation was credited to the work of the French chemist Antoine Lavoisier. However, recent work on the history of early modern chemistry considers the chemical revolution to consist of gradual changes in chemical theory and practice that emerged over a period of two centuries. The so-called scientific revolution took place during the sixteenth and seventeenth centuries whereas the chemical revolution took place during the seventeenth and eighteenth centuries.
 W
WIn Chinese alchemy, elixir poisoning refers to the toxic effects from elixirs of immortality that contained metals and minerals such as mercury and arsenic. The official Twenty-Four Histories record numerous Chinese emperors, nobles, and officials who died from taking elixirs in order to prolong their lifespans. The first emperor to die from elixir poisoning was likely Qin Shi Huang and the last was Yongzheng. Despite common knowledge that immortality potions could be deadly, fangshi and Daoist alchemists continued the elixir-making practice for two millennia.
 W
WThe cyclol hypothesis is the first structural model of a folded, globular protein. It was developed by Dorothy Wrinch in the late 1930s, and was based on three assumptions. Firstly, the hypothesis assumes that two peptide groups can be crosslinked by a cyclol reaction ; these crosslinks are covalent analogs of non-covalent hydrogen bonds between peptide groups. These reactions have been observed in the ergopeptides and other compounds. Secondly, it assumes that, under some conditions, amino acids will naturally make the maximum possible number of cyclol crosslinks, resulting in cyclol molecules and cyclol fabrics. These cyclol molecules and fabrics have never been observed. Finally, the hypothesis assumes that globular proteins have a tertiary structure corresponding to Platonic solids and semiregular polyhedra formed of cyclol fabrics with no free edges. Such "closed cyclol" molecules have not been observed either.
 W
WIn the history of the periodic table, Döbereiner's triads were an early attempt to sort the elements into some logical order by their physical properties. In 1817, a letter reported Johann Wolfgang Döbereiner's observations of the alkaline earths; namely, that strontium had properties that were intermediate to those of calcium and barium. By 1829, Döbereiner had found other groups of three elements whose physical properties were similarly related. He also noted that some quantifiable properties of elements in a triad followed a trend whereby the value of the middle element in the triad would be exactly or nearly predicted by taking the arithmetic mean of values for that property of the other two elements.
 W
WDyeing is the application of dyes or pigments on textile materials such as fibers, yarns, and fabrics with the goal of achieving color with desired color fastness. Dyeing is normally done in a special solution containing dyes and particular chemical material. Dye molecules are fixed to the fiber by absorption, diffusion, or bonding with temperature and time being key controlling factors. The bond between dye molecule and fiber may be strong or weak, depending on the dye used. Dyeing and printing are different applications; in printing, color is applied to a localized area with desired patterns. In dyeing, it is applied to the entire textile.
 W
WThe Dyson Perrins Laboratory is in the science area of the University of Oxford and was the main centre for research into organic chemistry of the University from its foundation in 1916 until its closure as a research laboratory in 2003. Until 2018, parts of the building were used as teaching laboratories in which undergraduate students were trained in practical organic chemistry.
 W
WIn the history of gunpowder there are a range of theories about the transmission of the knowledge of gunpowder and guns from China to the rest of the world.
 W
WAluminium metal is very rare in native form, and the process to refine it from ores is complex, so for most of human history it was unknown. However, the compound alum has been known since the 5th century BCE and was used extensively by the ancients for dyeing. During the Middle Ages, its use for dyeing made it a commodity of international commerce. Renaissance scientists believed that alum was a salt of a new earth; during the Age of Enlightenment, it was established that this earth, alumina, was an oxide of a new metal. Discovery of this metal was announced in 1825 by Danish physicist Hans Christian Ørsted, whose work was extended by German chemist Friedrich Wöhler.
 W
WThe history of biochemistry can be said to have started with the ancient Greeks who were interested in the composition and processes of life, although biochemistry as a specific scientific discipline has its beginning around the early 19th century. Some argued that the beginning of biochemistry may have been the discovery of the first enzyme, diastase, in 1833 by Anselme Payen, while others considered Eduard Buchner's first demonstration of a complex biochemical process alcoholic fermentation in cell-free extracts to be the birth of biochemistry. Some might also point to the influential work of Justus von Liebig from 1842, Animal chemistry, or, Organic chemistry in its applications to physiology and pathology, which presented a chemical theory of metabolism, or even earlier to the 18th century studies on fermentation and respiration by Antoine Lavoisier.
 W
WThe history of cosmetics spans at least 7,000 years and is present in almost every society on earth. Cosmetic body art is argued to have been the earliest form of a ritual in human culture. The evidence for this comes in the form of utilised red mineral pigments including crayons associated with the emergence of Homo sapiens in Africa.
 W
WGunpowder is the first explosive to have been developed. Popularly listed as one of the "Four Great Inventions" of China, it was discovered during the late Tang dynasty but the earliest record of a written formula appeared in the Song dynasty. Knowledge of gunpowder spread rapidly throughout Asia, the Middle East and Europe, possibly as a result of the Mongol conquests during the 13th century, with written formula for it appearing in the 1267 Opus Majus treatise by Roger Bacon and a 1280 treatise by Hasan al-Rammah. It was employed in warfare to some effect from at least the 10th century in weapons such as fire arrows, bombs, and the fire lance before the appearance of the gun. While the fire lance was eventually supplanted by the gun, other gunpowder weapons such as rockets and fire arrows continued to see use in China, Korea, India, and eventually Europe. Bombs too never ceased to develop and continued to progress into the modern day as grenades, mines, and other explosive implements. Gunpowder has also been used for non-military purposes such as fireworks for entertainment, or in explosives for mining and tunneling.
 W
WIn chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.
 W
WThe history of spectroscopy began in the 17th century. New designs in optics, specifically prisms, enabled systematic observations of the solar spectrum. Isaac Newton first applied the word spectrum to describe the rainbow of colors that combine to form white light. During the early 1800s, Joseph von Fraunhofer conducted experiments with dispersive spectrometers that enabled spectroscopy to become a more precise and quantitative scientific technique. Since then, spectroscopy has played and continues to play a significant role in chemistry, physics and astronomy. Fraunhofer observed and measured dark lines in the Sun's spectrum, which now bear his name although several of them were observed earlier by Wollaston.
 W
WThe periodic table is an arrangement of the chemical elements, structured by their atomic number, electron configuration and recurring chemical properties. In the basic form, elements are presented in order of increasing atomic number, in the reading sequence. Then, rows and columns are created by starting new rows and inderting blank cells, so that rows (periods) and columns (groups) show elements with recurring properties. For example, all elements in group (column) 18 are noble gases that hardly have a chemical reaction.
 W
WIatrochemistry is a branch of both chemistry and medicine. Having its roots in alchemy, iatrochemistry seeks to provide chemical solutions to diseases and medical ailments.
 W
WAlchemy and chemistry in Islam refers to the study of both traditional alchemy and early practical chemistry by Muslim scholars in the medieval Islamic world. The word alchemy was derived from the Arabic word كيمياء or kīmiyāʾ and may ultimately derive from the ancient Egyptian word kemi, meaning black.
 W
WThe Karlsruhe Congress was an international meeting of chemists held in Karlsruhe, Germany from 3 to 5 September 1860. It was the first international conference of chemistry worldwide.
 W
WA lime kiln is a kiln used for the calcination of limestone (calcium carbonate) to produce the form of lime called quicklime (calcium oxide). The chemical equation for this reaction isCaCO3 + heat → CaO + CO2
 W
WA limepit is either a place where limestone is quarried, or a man-made pit used to burn lime stones in the same way that modern-day kilns and furnaces constructed of brick are now used above ground for the calcination of limestone and by which quicklime is produced, an essential component in waterproofing and in wall plastering.
 W
WLute was a substance used to seal and affix apparatus employed in chemistry and alchemy, and to protect component vessels against heat damage by fire; it was also used to line furnaces. Lutation was thus the act of "cementing vessels with lute".
 W
WThe history of gaseous fuel, important for lighting, heating, and cooking purposes throughout most of the 19th century and the first half of the 20th century, began with the development of analytical and pneumatic chemistry in the 18th century. The manufacturing process for "synthetic fuel gases" typically consisted of the gasification of combustible materials, usually coal, but also wood and oil. The coal was gasified by heating the coal in enclosed ovens with an oxygen-poor atmosphere. The fuel gases generated were mixtures of many chemical substances, including hydrogen, methane, carbon monoxide and ethylene, and could be burnt for heating and lighting purposes. Coal gas, for example, also contains significant quantities of unwanted sulfur and ammonia compounds, as well as heavy hydrocarbons, and so the manufactured fuel gases needed to be purified before they could be used.
 W
WMauveine, also known as aniline purple and Perkin's mauve, was one of the first synthetic dyes. It was discovered serendipitously by William Henry Perkin in 1856 while he was attempting to create a cure for malaria. It is also among the first chemical dyes to have been mass-produced.
 W
WHelen Cecilia De Silver Abbott Michael was an American scientist who was among the first to "in a systematic way study the relation of chemical composition to species of plants and to plant growth." Michael theorized that the chemical composition of plants over the course of their development provided an illustration for the theory of evolution. She also was a student of Tufts, and later Harvard, and worked with organic chemist Arthur Michael, who she subsequently married.
 W
WThe Mystery of Matter: Search for the Elements is a 2014 American documentary miniseries, which premiered nationwide on August 19, 2015. The PBS documentary, in three-episodes of one hour each, was directed by Stephen Lyons and Muffie Meyer.
 W
WThe National Historic Chemical Landmarks program was launched by the American Chemical Society in 1992 to recognize significant achievements in the history of chemistry and related professions. The program celebrates the centrality of chemistry. The designation of such generative achievements in the history of chemistry demonstrates how chemists have benefited society by fulfilling the ACS vision: Improving people's lives through the transforming power of chemistry. The program occasionally designates International Historic Chemical Landmarks to commemorate "chemists and chemistry from around the world that have had a major impact in the United States".
 W
WThe phlogiston theory is a superseded scientific theory that postulated the existence of a fire-like element called phlogiston contained within combustible bodies and released during combustion. The name comes from the Ancient Greek φλογιστόν phlogistón, from φλόξ phlóx (flame). The idea was first proposed in 1667 by Johann Joachim Becher and later put together more formally by Georg Ernst Stahl. Phlogiston theory attempted to explain chemical processes of weight increase such as combustion and rusting, now collectively known as oxidation, and was abandoned before the end of the 18th century following experiments by Antoine Lavoisier and others. Phlogiston theory led to experiments which ultimately concluded with the discovery of oxygen.
 W
WIn the history of science, pneumatic chemistry is an area of scientific research of the seventeenth, eighteenth, and early nineteenth centuries. Important goals of this work were an understanding of the physical properties of gases and how they relate to chemical reactions and, ultimately, the composition of matter. The rise of phlogiston theory, and its replacement with the description of oxygen as a component of the atmosphere and a factor in combustion, were addressed in the era of pneumatic chemistry.
 W
WTheodore William Richards was the first American scientist to receive the Nobel Prize in Chemistry, earning the award "in recognition of his exact determinations of the atomic weights of a large number of the chemical elements."
 W
WThe Royal College of Chemistry (RCC) was a college originally based on Oxford Street in central London, England. It operated between 1845 and 1872.
 W
WThe Science History Institute is an institution that preserves and promotes understanding of the history of science. Located in Philadelphia, Pennsylvania, it includes a library, museum, archive, research center and conference center.
 W
WThe Society for the History of Alchemy and Chemistry, founded as the Society for the Study of Alchemy and Early Chemistry in 1935, holds biennial meetings and a yearly Graduate Workshop, publishes the journal Ambix and a biennial newsletter Chemical Intelligence, and offers prizes and grants to scholars. It has a worldwide membership.
 W
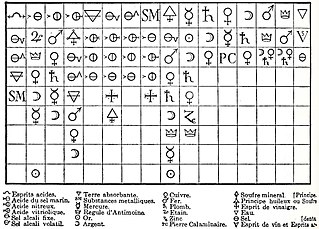
WThe Tabula Affinitatum is a table of chemical affinities between substances.
 W
WThis timeline of chemistry lists important works, discoveries, ideas, inventions, and experiments that significantly changed humanity's understanding of the modern science known as chemistry, defined as the scientific study of the composition of matter and of its interactions. The history of chemistry in its modern form arguably began with the Irish scientist Robert Boyle, though its roots can be traced back to the earliest recorded history.
 W
WTyrocinium Chymicum was a published set of chemistry lecture notes started by Jean Beguin in 1610 in Paris, France. It has been cited as the first chemistry textbook. Many of the preparations were pharmaceutical in nature.