 W
WThe pharmaceutical industry discovers, develops, produces, and markets drugs or pharmaceutical drugs for use as medications to be administered to patients, with the aim to cure them, vaccinate them, or alleviate the symptoms. Pharmaceutical companies may deal in generic or brand medications and medical devices. They are subject to a variety of laws and regulations that govern the patenting, testing, safety, efficacy and marketing of drugs.
 W
WArven Pharmaceuticals is a Turkish pharmaceutical corporation headquartered in Istanbul established as a subsidiary of Toksöz Group in 2013. Arven’s primary focus is development and production of high-technology inhaler and biotechnology products. The company is specialized on difficult to make products and strives to develop quality products. Arven is the first Turkish company developing biosimilars for global markets, including USA and EU.
 W
WBad Pharma: How Drug Companies Mislead Doctors and Harm Patients is a book by the British physician and academic Ben Goldacre about the pharmaceutical industry, its relationship with the medical profession, and the extent to which it controls academic research into its own products. It was published in the UK in September 2012 by the Fourth Estate imprint of HarperCollins, and in the United States in February 2013 by Faber and Faber.
 W
WBig Pharma: How the World's Biggest Drug Companies Control Illness is a 2006 book by British journalist Jacky Law. The book examines how major pharmaceutical companies determine which health care problems are publicised and researched.
 W
WA botanical drug is defined in the United States Federal Food, Drug, and Cosmetic Act as a botanical product that is marketed as diagnosing, mitigating, treating, or curing a disease; a botanical product in turn, is a finished, labeled product that contains ingredients from plants. Chemicals that are purified from plants, like paclitaxel, and highly purified products of industrial fermentation, like biopharmaceuticals, are not considered to be botanical products.
 W
WIn the manufacture of pharmaceuticals, encapsulation refers to a range of dosage forms—techniques used to enclose medicines—in a relatively stable shell known as a capsule, allowing them to, for example, be taken orally or be used as suppositories. The two main types of capsules are:Hard-shelled capsules, which contain dry, powdered ingredients or miniature pellets made by e.g. processes of extrusion or spheronization. These are made in two halves: a smaller-diameter “body” that is filled and then sealed using a larger-diameter “cap”. Soft-shelled capsules, primarily used for oils and for active ingredients that are dissolved or suspended in oil.
 W
WChemogenomics, or chemical genomics, is the systematic screening of targeted chemical libraries of small molecules against individual drug target families with the ultimate goal of identification of novel drugs and drug targets. Typically some members of a target library have been well characterized where both the function has been determined and compounds that modulate the function of those targets have been identified. Other members of the target family may have unknown function with no known ligands and hence are classified as orphan receptors. By identifying screening hits that modulate the activity of the less well characterized members of the target family, the function of these novel targets can be elucidated. Furthermore, the hits for these targets can be used as a starting point for drug discovery. The completion of the human genome project has provided an abundance of potential targets for therapeutic intervention. Chemogenomics strives to study the intersection of all possible drugs on all of these potential targets.
 W
WClearTrial is a multinational software developer headquartered in Chicago, Illinois, USA. The company develops and markets a software as a service (SaaS) system designed for biopharmaceutical and medical device manufacturers for the planning, outsourcing, and tracking of clinical trials.
 W
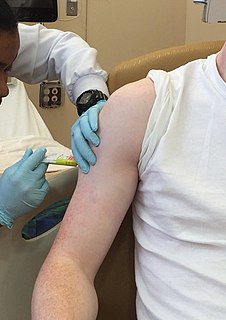
WClinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.
 W
WA cold chain is a temperature-controlled supply chain. An unbroken cold chain is an uninterrupted series of refrigerated production, storage and distribution activities, along with associated equipment and logistics, which maintain quality via a desired low-temperature range. It is used to preserve and to extend and ensure the shelf life of products, such as fresh agricultural produce, seafood, frozen food, photographic film, chemicals, and pharmaceutical products. Such products, during transport and when in transient storage, are sometimes called cool cargo. Unlike other goods or merchandise, cold chain goods are perishable and always en route towards end use or destination, even when held temporarily in cold stores and hence commonly referred to as "cargo" during its entire logistics cycle.
 W
WCorporate Crime in the Pharmaceutical Industry is a 1984 book on corporate crime and the pharmaceutical industry by criminologist John Braithwaite.
 W
WCOVID‑19 drug development is the research process to develop preventative therapeutic prescription drugs that would alleviate the severity of coronavirus disease 2019 (COVID‑19). Internationally by November 2020, several hundred drug companies, biotechnology firms, university research groups, and health organizations were developing over 500 potential therapies for COVID‑19 disease in various stages of preclinical or clinical research.
 W
WDisease mongering is a term for the practice of widening the diagnostic boundaries of illnesses and aggressively promoting their public awareness in order to expand the markets for treatment. Among the entities benefiting from selling and delivering treatments are pharmaceutical companies, physicians, alternative practitioners and other professional or consumer organizations. It is distinct from the promulgation of bogus or unrecognised diagnoses.
 W
WA drug coupon is a coupon intended to help consumers save money on pharmaceutical drugs. They are offered by drug companies or distributed to consumers via doctors and pharmacists, and most can be obtained online. There are drug coupons for drugs from many categories such as cholesterol, acne, migraine, allergies, etc.
 W
WDrug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug.
 W
WDrug nomenclature is the systematic naming of drugs, especially pharmaceutical drugs. In the majority of circumstances, drugs have 3 types of names: chemical names, the most important of which is the IUPAC name; generic or nonproprietary names, the most important of which are the International Nonproprietary Names (INNs); and trade names, which are brand names. Generic names for drugs are nowadays constructed out of affixes and stems that classify the drugs into different categories and also separate drugs within categories. A marketed drug might also have a company code or compound code.
 W
WPharmaceutical packaging is the packages and the packaging processes for pharmaceutical preparations. It involves all of the operations from production through drug distribution channels to the end consumer.
 W
WEminent College of Pharmaceutical Technology (ECPT) is a private pharmacy college located in Moshpukr, Barbaria, Barasat, West Bengal, about 4.8 kilometers from the Barasat Junction railway station and 11.4 kilometers from the Barrackpore Railway Station. It was established in 2017 with its first batch of D. Pharm students and B. Pharm was started next year. It offers a 2 year Diploma in Pharmacy and 4 year Bachelor of Pharmacy courses (Degree). The college is affiliated to Maulana Abul Kalam Azad University of Technology and West Bengal State Council of Technical and Vocational Education and Skill Development and approved by All India Council for Technical Education (AICTE) and PCI
 W
WThe environmental effect of pharmaceuticals and personal care products (PPCPs) is currently being widely investigated. PPCPs include substances used by individuals for personal health or cosmetic reasons and the products used by agribusiness to boost growth or health of livestock. More than twenty million tons of PPCPs are produced every year.
 W
WThe term Environmental persistent pharmaceutical pollutants (EPPP) was first suggested in the nomination in 2010 of pharmaceuticals and environment as an emerging issue in a Strategic Approach to International Chemicals Management (SAICM) by the International Society of Doctors for the Environment (ISDE). The occurring problems from EPPPs are in parallel explained under environmental impact of pharmaceuticals and personal care products (PPCP). The European Union summarizes pharmaceutical residues with the potential of contamination of water and soil together with other micropollutants under “priority substances”.
 W
WAn epedigree is an electronic document which provides data on the history of a particular batch of a drug. It satisfies the requirement for a drug pedigree while using a convenient electronic form.
 W
WFire in the Blood is a 2013 documentary film by Dylan Mohan Gray depicting the intentional obstruction of access to low-cost antiretroviral drugs used in the treatment of HIV/AIDS to people in Africa and other parts the global south, driven by multinational pharmaceutical companies holding patent monopolies and various Western governments consistently doing these companies' bidding. The film details how the battle against this genocidal blockade, estimated to have resulted in no less than ten- to twelve million completely unnecessary deaths, was fought and won.
 W
WFirst Databank (FDB) is a major provider of drug and medical device databases that help inform healthcare professionals to make decisions. FDB partners with information system developers to deliver useful medication- and medical device-related information to clinicians, business associates, and patients. FDB is part of Hearst and the Hearst Health network.
 W
WA generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active chemical substance is the same, the medical profile of generics is believed to be equivalent in performance. A generic drug has the same active pharmaceutical ingredient (API) as the original, but it may differ in some characteristics such as the manufacturing process, formulation, excipients, color, taste, and packaging.
 W
WGeneric pharmaceutical price decay is what happens once the originator brand has lost its patent exclusivity and generic versions of the originator brand have been launched.
 W
WGood manufacturing practices (GMP) are the practices required in order to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices. These guidelines provide minimum requirements that a manufacturer must meet to assure that their products are consistently high in quality, from batch to batch, for their intended use. The rules that govern each industry may differ significantly; however, the main purpose of GMP is always to prevent harm from occurring to the end user. Additional tenets include ensuring the end product is free from contamination, that it is consistent in its manufacture, that its manufacture has been well documented, that personnel are well trained, and that the product has been checked for quality more than just at the end phase. GMP is typically ensured through the effective use of a quality management system (QMS).
 W
WIn biology and other experimental sciences, an in silico experiment is one performed on computer or via computer simulation. The phrase is pseudo-Latin for "in silicon", referring to silicon in computer chips. It was coined in 1987 as an allusion to the Latin phrases in vivo, in vitro, and in situ, which are commonly used in biology. The latter phrases refer, respectively, to experiments done in living organisms, outside living organisms, and where they are found in nature.
 W
WThe Innovative Medicines Initiative (IMI) is a European initiative to improve the competitive situation of the European Union in the field of pharmaceutical research. The IMI is a joint initiative of the DG Research of the European Commission, representing the European Communities, and the European Federation of Pharmaceutical Industries and Associations (EFPIA). IMI is laid out as a Joint Technology Initiative within the Seventh Framework Programme. Michel Goldman was the first executive director, from September 2009 until December 2014.
 W
WLipinski's rule of five, also known as Pfizer's rule of five or simply the rule of five (RO5), is a rule of thumb to evaluate druglikeness or determine if a chemical compound with a certain pharmacological or biological activity has chemical properties and physical properties that would make it a likely orally active drug in humans. The rule was formulated by Christopher A. Lipinski in 1997, based on the observation that most orally administered drugs are relatively small and moderately lipophilic molecules.
 W
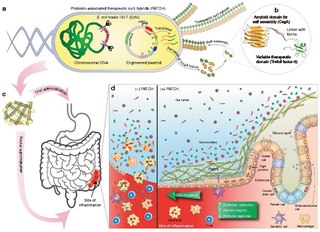
WA living medicine is a type of biologic that consists of a living organism that is used to treat a disease. This usually takes the form of a cell or a virus that has been genetically engineered to possess therapeutic properties that is injected into a patient. Perhaps the oldest use of a living medicine is the use of leeches for bloodletting, though living medicines have advanced tremendously since this time.
 W
WThe pharmaceutical lobby refers to the representatives of pharmaceutical drug and biomedicine companies who engage in lobbying in favour of the pharmaceutical industry and its products.
 W
WThe Medical Products Agency is the government agency in Sweden responsible for regulation and surveillance of the development, manufacturing and sale of medicinal drugs, medical devices and cosmetics.
 W
WA package insert is a document included in the package of a medication that provides information about that drug and its use. For prescription medications, the insert is technical, providing information for medical professionals about how to prescribe the drug. Package inserts for prescription drugs often include a separate document called a "patient package insert" with information written in plain language intended for the end-user -- the person who will take the drug or give the drug to another person, such as a minor. Inserts for over-the-counter medications are also written plainly.
 W
WThe Mexican barbasco trade was the trade of the diosgenin-rich yam species Dioscorea mexicana, Dioscorea floribunda and Dioscorea composita which emerged in Mexico in the 1950s as part of the Mexican steroid industry. The trade consisted in Mexican campesinos harvesting the root in the jungle, selling it to middlemen who brought it to processing plants where the root was fermented and the diosgenin extracted and sold to pharmaceutical companies such as Syntex who used it to produce synthetic hormones.
 W
WAt equilibrium, the relationship between water content and equilibrium relative humidity of a material can be displayed graphically by a curve, the so-called moisture sorption isotherm. For each humidity value, a sorption isotherm indicates the corresponding water content value at a given, constant temperature. If the composition or quality of the material changes, then its sorption behaviour also changes. Because of the complexity of sorption processes, the isotherms cannot be determined explicitly by calculation, but must be recorded experimentally for each product.
 W
WThe Food and Drug Administration (FDA)'s New Drug Application (NDA) is the vehicle in the United States through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing. Some 30% or less of initial drug candidates proceed through the entire multi-year process of drug development, concluding with an approved NDA, if successful.
 W
WThe Newhouse Research Site is a drug research facility situated 15 miles (24 km) east of Glasgow in central Scotland. It is located beside the M8 motorway in Newhouse, North Lanarkshire. The site is an early drug discovery research centre with a track record of generating a succession of products in the areas of anaesthesia and psychiatry. In 2007, the Royal Society of Chemistry Malcolm Campbell Memorial Prize was awarded to researchers for its work on a new anaesthesia drug, sugammadex. It currently employs 250 scientists across a range of disciplines including medicinal chemistry, molecular biology and drug metabolism. The site is currently the largest private drug discovery centre in Scotland, and one of the biggest in the UK.
 W
WThe osmotic-controlled release oral delivery system (OROS) is an advanced controlled release oral drug delivery system in the form of a rigid tablet with a semi-permeable outer membrane and one or more small laser drilled holes in it. As the tablet passes through the body, water is absorbed through the semipermeable membrane via osmosis, and the resulting osmotic pressure is used to push the active drug through the laser drilled opening(s) in the tablet and into the gastrointestinal tract. OROS is a trademarked name owned by ALZA Corporation, which pioneered the use of osmotic pumps for oral drug delivery.
 W
WA medication is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management.
 W
WThe Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (PIC/S) are two international instruments between countries and pharmaceutical inspection authorities. The PIC/S is meant as an instrument to improve co-operation in the field of Good Manufacturing Practices between regulatory authorities and the pharmaceutical industry.
 W
WDrug manufacturing is the process of industrial-scale synthesis of pharmaceutical drugs as part of the pharmaceutical industry. The process of drug manufacturing can be broken down into a series of unit operations, such as milling, granulation, coating, tablet pressing, and others.
 W
WIn drug development, preclinical development, also named preclinical studies and nonclinical studies, is a stage of research that begins before clinical trials can begin, and during which important feasibility, iterative testing and drug safety data are collected, typically in laboratory animals.
 W
WSinecatechins is a specific water extract of green tea leaves from Camellia sinensis that is the active ingredient in an ointment approved by the FDA in 2006 as a botanical drug to treat genital warts. Sinecatechins are mostly catechins, 55% of which is epigallocatechin gallate. It was the first botanical drug approved by the US FDA.
 W
WThe Solidarity trial for treatments is a multinational Phase III-IV clinical trial organized by the World Health Organization (WHO) and partners to compare four untested treatments for hospitalized people with severe COVID-19 illness. The trial was announced 18 March 2020, and as of 1 July, nearly 5,500 patients in 21 countries had been recruited to participate in the trial.
 W
WSpray drying is a method of producing a dry powder from a liquid or slurry by rapidly drying with a hot gas. This is the preferred method of drying of many thermally-sensitive materials such as foods and pharmaceuticals. A consistent particle size distribution is a reason for spray drying some industrial products such as catalysts. Air is the heated drying medium; however, if the liquid is a flammable solvent such as ethanol or the product is oxygen-sensitive then nitrogen is used.
 W
WStevanato Group is an Italian multinational company headquartered in Piombino Dese, Padua – Italy. Founded in 1949, it is also active in the glass tube forming technology and inspection systems sector.
 W
WA tablet is a pharmaceutical oral dosage form or solid unit dosage form. Tablets may be defined as the solid unit dosage form of medicament or medicaments with suitable excipients. It comprises a mixture of active substances and excipients, usually in powder form, pressed or compacted from a powder into a solid dose.
 W
WA tablet press is a mechanical device that compresses powder into tablets of uniform size and weight. A tablet press can be used to manufacture tablets of a wide variety of materials, including pharmaceuticals, nutraceuticals, cleaning products, industrial pellets and cosmetics. To form a tablet, the granulated powder material must be metered into a cavity formed by two punches and a die, and then the punches must be pressed together with great force to fuse the material together.
 W
WTigers is a 2014 Indian drama film directed by Danis Tanovic, and produced as a joint production between Cinema Time, French production company ASAP Films and Sikhya Entertainment.
 W
WThe UCSF Library is the library of the University of California, San Francisco. It is one of the world's foremost libraries in the health sciences.
 W
WModern pharmaceutical manufacturing techniques frequently rely upon biotechnology.