 W
WOzone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone around Earth's polar regions. The latter phenomenon is referred to as the ozone hole. There are also springtime polar tropospheric ozone depletion events in addition to these stratospheric events.
 W
WBromochloromethane or methylene bromochloride and Halon 1011 is a mixed halomethane. It is a heavy low-viscosity liquid with refractive index 1.4808.
 W
WBromodifluoromethane or Halon 1201 or FC-22B1 is a gaseous trihalomethane or a hydrobromofluorocarbon.
 W
WBromofluorocarbons (BFCs) are molecules based on carbon, bromine, and fluorine. The most common use has traditionally been in fire suppression systems. The brand name "Halon" is frequently used interchangeably for BFCs. However, not all Halons are technically BFCs.
 W
WChlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated paraffin hydrocarbons that contain only carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane. They are also commonly known by the DuPont brand name Freon.
 W
WChloropentafluoroethane is a chlorofluorocarbon (CFC) once used as a refrigerant and also known as R-115 and CFC-115. Its production and consumption has been banned since 1 January 1996 under the Montreal Protocol because of its high ozone depletion potential and very long lifetime when released into the environment. CFC-115 is also a potent greenhouse gas.
 W
WThe ozone–oxygen cycle is the process by which ozone is continually regenerated in Earth's stratosphere, converting ultraviolet radiation (UV) into heat. In 1930 Sydney Chapman resolved the chemistry involved. The process is commonly called the Chapman cycle by atmospheric scientists.
 W
WDibromodifluoromethane is a mixed halomethane. It is a colorless non-flammable liquid.
 W
WDibromofluoromethane is a mixed halomethane. It is soluble in alcohol, acetone, benzene and chloroform.
 W
WDichloromethane (DCM or methylene chloride) is an organochloride compound with the formula CH2Cl2. This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with water, it is polar, and miscible with many organic solvents.
 W
WSeptember 16 was designated by the United Nations General Assembly as the International Day for the Preservation of the Ozone Layer. This designation had been made on December 19, 2000, in commemoration of the date, in 1987, on which nations signed the Montreal Protocol on Substances that Deplete the Ozone Layer. In 1994, the UN General Assembly proclaimed 16 September the International Day for the Preservation of the Ozone Layer, commemorating the date of the signing, in 1987, of the Montreal Protocol on Substances that Deplete the Ozone Layer. The closure of the hole in the ozone layer was observed 30 years after the protocol was signed. Due to the nature of the gases responsible for ozone depletion their chemical effects are expected to continue for between 50 and 100 years.
 W
WThe ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 parts per million of ozone, while the average ozone concentration in Earth's atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 15 to 35 kilometers (9 to 22 mi) above Earth, although its thickness varies seasonally and geographically.
 W
WThe Montreal Protocol is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion. It was agreed on 16 September 1987, and entered into force on 1 January 1989. Since then, it has undergone nine revisions, in 1990 (London), 1991 (Nairobi), 1992 (Copenhagen), 1993 (Bangkok), 1995 (Vienna), 1997 (Montreal), 1998 (Australia), 1999 (Beijing) and 2016 (Kigali) As a result of the international agreement, the ozone hole in Antarctica is slowly recovering. Climate projections indicate that the ozone layer will return to 1980 levels between 2050 and 2070. Due to its widespread adoption and implementation it has been hailed as an example of successful international co-operation, with Kofi Annan quoted as saying that "perhaps the single most successful international agreement to date has been the Montreal Protocol". In comparison, effective burden sharing and solution proposals mitigating regional conflicts of interest have been among the success factors for the ozone depletion challenge, where global regulation based on the Kyoto Protocol has failed to do so. In this case of the ozone depletion challenge, there was global regulation already being installed before a scientific consensus was established. Also, overall public opinion was convinced of possible imminent risks.
 W
WThe ozone monitoring instrument (OMI) is a nadir-viewing visual and ultraviolet spectrometer aboard the NASA Aura spacecraft. Aura flies in formation about 15 minutes behind Aqua, both of which orbit the earth in a polar Sun-synchronous pattern. Aura was launched on July 15, 2004, and OMI has collected data since August 9, 2004. OMI can distinguish between aerosol types, such as smoke, dust, and sulfates, and can measure cloud pressure and coverage, which provide data to derive tropospheric ozone. OMI follows in the heritage of TOMS, SBUV, GOME, SCIAMACHY, and GOMOS. OMI measurements cover a spectral region of 264–504 nm (nanometers) with a spectral resolution between 0.42 nm and 0.63 nm and a nominal ground footprint of 13 × 24 km2 at nadir. The Aura satellite orbits at an altitude of 705 km in a sun-synchronous polar orbit with an exact 16-day repeat cycle and wit h a local equator crossing time of 13. 45 on the ascending node. The orbital inclination is 98.1 degrees, providing latitudinal coverage from 82° N to 82° S. It is a wide-field-imaging spectrometer with a 114° across-track viewing angle range that provides a 2600 km wide swath, enabling measurements with a daily global coverage. OMI is continuing the TOMS record for total ozone and other atmospheric parameters related to ozone chemistry and climate.
 W
WPolar stratospheric clouds (PSCs) are clouds in the winter polar stratosphere at altitudes of 15,000–25,000 m (49,000–82,000 ft). They are best observed during civil twilight, when the Sun is between 1 and 6 degrees below the horizon, as well as in winter and in more northerly latitudes. One main type of PSC is made up mostly of supercooled droplets of water and nitric acid and is implicated in the formation of ozone holes. The other main type consists only of ice crystals which are not harmful. This type of PSC is also referred to as nacreous.
 W
WThe Scientific Assessment of Ozone Depletion is a sequence of reports sponsored by WMO/UNEP. The most recent is the 2018 report. The reports were set up to inform the Montreal Protocol and amendments about ozone depletion.
 W
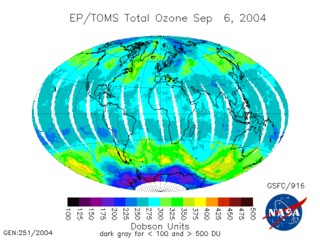
WThe Total Ozone Mapping Spectrometer (TOMS) was a NASA satellite instrument, specifically a spectrometer, for measuring the ozone layer. Of the five TOMS instruments which were built, four entered successful orbit. The satellites carrying TOMS instruments were:Nimbus 7; launched October 24, 1978. Operated until 1 August 1994. Carried TOMS instrument number 1. Meteor-3-5; launched 15 August 1991. Operated until December 1994. Was the first and last Soviet satellite to carry a USA made instrument. Carried TOMS instrument number 2. ADEOS I; launched 17 August 1996. Operated until 30 June 1997. Mission was cut short by a spacecraft failure. TOMS-Earth Probe; launched on July 2, 1996. Operated until 2 December 2006. Carried TOMS instrument number 3. QuikTOMS; launched 21 September 2001. Suffered launch failure and did not enter orbit.
 W
WDuring springtime in the polar regions of Earth, unique photochemistry converts inert halide salt ions into reactive halogen species that episodically deplete ozone in the atmospheric boundary layer to near zero levels. Since their discovery in the late 1980s, research on these ozone depletion events (ODEs) has shown the central role of bromine photochemistry. Due to the autocatalytic nature of the reaction mechanism, it has been called bromine explosion. It is still not fully understood how salts are transported from the ocean and oxidized to become reactive halogen species in the air. Other halogens are also activated through mechanisms coupled to bromine chemistry. The main consequence of halogen activation is chemical destruction of ozone, which removes the primary precursor of atmospheric oxidation, and generation of reactive halogen atoms/oxides that become the primary oxidizing species. The different reactivity of halogens as compared to OH and ozone has broad impacts on atmospheric chemistry, including near complete removal and deposition of mercury, alteration of oxidation fates for organic gases, and export of bromine into the free troposphere. Recent changes in the climate of the Arctic and state of the Arctic sea ice cover are likely to have strong effects on halogen activation and ODEs.