 W
WA cyanide is a chemical compound that contains the group C≡N. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
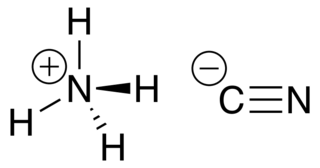 W
WAmmonium cyanide is an unstable inorganic compound with the formula NH4CN.
 W
WBarium cyanide is a chemical compound with the formula Ba(CN)2. It is synthesized by the reaction of hydrogen cyanide and barium hydroxide in water or petroleum ether. This white crystalline salt reacts with water and carbon dioxide in air slowly, producing highly toxic hydrogen cyanide gas.
 W
WCadmium cyanide is an inorganic compound with the formula Cd(CN)2. It is a white crystalline compound that is used in electroplating. It is very toxic, along with other cadmium and cyanide compounds.
 W
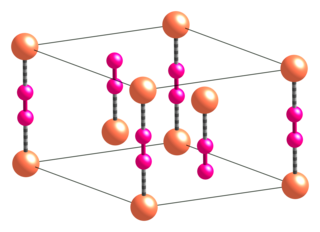
WCalcium cyanide also known as black cyanide, is the calcium salt of cyanide, an inorganic compound with the formula Ca(CN)2. The pure form is a white solid, although rarely observed; commercial samples can be black-gray. It hydrolyses readily (even in moist air) to release hydrogen cyanide. Like other similar cyanides it is very toxic.
 W
WCopper(I) cyanide is an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a catalyst, in electroplating copper, and as a reagent in the preparation of nitriles.
 W
WCyanide poisoning is poisoning that results from exposure to any of a number of forms of cyanide. Early symptoms include headache, dizziness, fast heart rate, shortness of breath, and vomiting. This phase may then be followed by seizures, slow heart rate, low blood pressure, loss of consciousness, and cardiac arrest. Onset of symptoms usually occurs within a few minutes. Some survivors may have long-term neurological problems.
 W
WThe cyano radical is a radical with molecular formula CN, sometimes written ·CN. The cyanido radical was one of the first detected molecules in the interstellar medium. Its detection and analysis was influential in astrochemistry. The first discovery was performed with a coudé spectrograph, which was made famous and credible due to this detection. ·CN has been observed in both diffuse clouds and dense clouds. Usually, CN is detected in regions with hydrogen cyanide, hydrogen isocyanide, and HCHN+, since it is involved in the creation and destruction of these species.
 W
W4-Cyano-3-(trifluoromethyl)aniline, also known as 4-amino-2-(trifluoromethyl)benzonitrile, is a cyanated and trifluoromethylated derivative of aniline. It is the starting material in one of the chemical syntheses of the nonsteroidal antiandrogen bicalutamide.
 W
WCyanogen azide, N3CN or CN4, is an azide compound of carbon and nitrogen which is an oily, colourless liquid at room temperature. It is a highly explosive chemical that is soluble in most organic solvents, and normally handled in dilute solution in this form. It was first synthesised by F.D. Marsh at DuPont in the early 1960s.
 W
WCyanogen fluoride is an inorganic linear compound which consists of a fluorine in a single bond with carbon, and a nitrogen in a triple bond with carbon. It is a toxic and explosive gas at room temperature. It is used in organic synthesis and can be produced by pyrolysis of cyanuric fluoride or by fluorination of cyanogen.
 W
WDiethylaluminum cyanide ("Nagata's reagent") is the organoaluminum compound with formula ((C2H5)2AlCN)n. This colorless compound is usually handled as a solution in toluene. It is a reagent for the hydrocyanation of α,β-unsaturated ketones.
 W
WDimethylamidophosphoric dicyanide is an important chemical for the final process of synthesizing Tabun, a nerve agent used as a chemical weapon.
 W
WDiphenylcyanoarsine, also called Clark 2 by the Germans, was discovered in 1918 by Sturniolo and Bellinzoni and shortly thereafter used like the related Clark 1 gas by the Germans for chemical warfare in the First World War. The substance causes nausea, vomiting and headaches. It can subsequently lead to e.g. pulmonary edema.
 W
WLithium cyanide is an inorganic compound with the chemical formula LiCN. It is a white, hygroscopic, water-soluble salt that finds only niche uses.
 W
WMercury(II) cyanide, also known as mercuric cyanide, is a compound of mercury. It is an odorless, toxic white powder. It is highly soluble in polar solvents such as water, alcohol, and ammonia; slightly soluble in ether; and insoluble in benzene and other hydrophobic solvents.
 W
WPotassium cyanide is a compound with the formula KCN. This colorless crystalline salt, similar in appearance to sugar, is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewellery for chemical gilding and buffing.
 W
WPrussian blue is a dark blue pigment produced by oxidation of ferrous ferrocyanide salts. It has the chemical formula FeIII4[FeII(CN)6]3. Turnbull's blue is chemically identical, but is made from different reagents, and its slightly different color stems from different impurities.
 W
WSodium cyanide is a poisonous compound with the formula NaCN. It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its high reactivity toward metals. It is a moderately strong base. When treated with acid, it forms the toxic gas hydrogen cyanide:NaCN + H2SO4 → HCN + NaHSO4
 W
WZinc cyanide is the inorganic compound with the formula Zn(CN)2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the synthesis of organic compounds.
 W
WZyklon B was the trade name of a cyanide-based pesticide invented in Germany in the early 1920s. It consisted of hydrogen cyanide, as well as a cautionary eye irritant and one of several adsorbents such as diatomaceous earth. The product is notorious for its use by Nazi Germany during the Holocaust to murder approximately 1.1 million people in gas chambers installed at Auschwitz-Birkenau, Majdanek, and other extermination camps.