 W
WAlbumin is a family of globular proteins, the most common of which are the serum albumins. All the proteins of the albumin family are water-soluble, moderately soluble in concentrated salt solutions, and experience heat denaturation. Albumins are commonly found in blood plasma and differ from other blood proteins in that they are not glycosylated. Substances containing albumins are called albuminoids.
 W
WAlpha globulins are a group of globular proteins in plasma that are highly mobile in alkaline or electrically charged solutions. They inhibit certain blood proteases and show significant inhibitor activity.
 W
WAquaporin 3 is the protein product of the human AQP3 gene. It is found in the basolateral cell membrane of principal collecting duct cells and provides a pathway for water to exit these cells. Aquaporin 3 is also permeable to glycerol, ammonia, urea, and hydrogen peroxide. It is expressed in various tissues including the skin, respiratory tract, and kidneys as well as various types of cancers. In the kidney, aquaproin 3 is unresponsive to vasopressin, unlike Aquaporin 2. This protein is also a determinant for the GIL blood group system.
 W
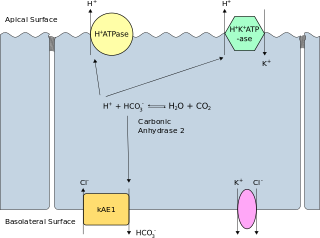
WBand 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the SLC4A1 gene in humans.
 W
WBeta globulins are a group of globular proteins in plasma that are more mobile in alkaline or electrically charged solutions than gamma globulins, but less mobile than alpha globulins.
 W
WBovine serum albumin is a serum albumin protein derived from cows. It is often used as a protein concentration standard in lab experiments.
 W
WCholesteryl ester transfer protein (CETP), also called plasma lipid transfer protein, is a plasma protein that facilitates the transport of cholesteryl esters and triglycerides between the lipoproteins. It collects triglycerides from very-low-density (VLDL) or low-density lipoproteins (LDL) and exchanges them for cholesteryl esters from high-density lipoproteins (HDL), and vice versa. Most of the time, however, CETP does a heteroexchange, trading a triglyceride for a cholesteryl ester or a cholesteryl ester for a triglyceride.
 W
WOvotransferrin (conalbumin) is a glycoprotein of egg white albumen. Egg white albumen is composed of multiple proteins, of which ovotransferrin is the most heat reliable. It has a molecular weight of 76,000 daltons and contains about 700 amino acids. Ovotransferrin makes up approximately 13% of egg albumen. As a member of the transferrin and metalloproteinase family, ovotransferrin has been found to produce heat shock proteins. When these heat shock proteins are induced in the skin, they provide protection against cold stress and other environmental stresses.
 W
WErythroferrone is a protein hormone encoded in humans by the ERFE gene. Erythroferrone is produced by erythroblasts, inhibits the production of hepcidin in the liver, and so increases the amount of iron available for hemoglobin synthesis. Skeletal muscle secreted ERFE has been shown to maintain systemic metabolic homeostasis.
 W
WFetuins are blood proteins that are made in the liver and secreted into the bloodstream. They belong to a large group of binding proteins mediating the transport and availability of a wide variety of cargo substances in the bloodstream. Fetuin-A is a major carrier protein of free fatty acids in the circulation. The best known representative of carrier proteins is serum albumin, the most abundant protein in the blood plasma of adult animals. Fetuin is more abundant in fetal blood, hence the name "fetuin". Fetal bovine serum contains more fetuin than albumin, while adult serum contains more albumin than fetuin.
 W
WFibrin is a fibrous, non-globular protein involved in the clotting of blood. It is formed by the action of the protease thrombin on fibrinogen, which causes it to polymerize. The polymerized fibrin, together with platelets, forms a hemostatic plug or clot over a wound site.
 W
WFibrinogen is a glycoprotein complex, made in the liver, that circulates in the blood of all vertebrates. During tissue and vascular injury, it is converted enzymatically by thrombin to fibrin and then to a fibrin-based blood clot. Fibrin clots function primarily to occlude blood vessels to stop bleeding. Fibrin also binds and reduces the activity of thrombin. This activity, sometimes referred to as antithrombin I, limits clotting. Fibrin also mediates blood platelet and endothelial cell spreading, tissue fibroblast proliferation, capillary tube formation, and angiogenesis and thereby promotes revascularization and wound healing.
 W
WFibulin (FY-beau-lin) is the prototypic member of a multigene family, currently with seven members. Fibulin-1 is a calcium-binding glycoprotein. In vertebrates, fibulin-1 is found in blood and extracellular matrices. In the extracellular matrix, fibulin-1 associates with basement membranes and elastic fibers. The association with these matrix structures is mediated by its ability to interact with numerous extracellular matrix constituents including fibronectin, proteoglycans, laminins and tropoelastin. In blood, fibulin-1 binds to fibrinogen and incorporates into clots.
 W
WGamma globulins are a class of globulins, identified by their position after serum protein electrophoresis. The most significant gamma globulins are immunoglobulins (antibodies), although some immunoglobulins are not gamma globulins, and some gamma globulins are not immunoglobulins.
 W
WHaptoglobin is the protein that in humans is encoded by the HP gene. In blood plasma, haptoglobin binds to free hemoglobin, compared to hemopexin that binds to free heme, released from erythrocytes with high affinity, and thereby inhibits its deleterious oxidative activity. The haptoglobin-hemoglobin complex will then be removed by the reticuloendothelial system.
 W
WHemocyanins (also spelled haemocyanins and abbreviated Hc) are proteins that transport oxygen throughout the bodies of some invertebrate animals. These metalloproteins contain two copper atoms that reversibly bind a single oxygen molecule (O2). They are second only to hemoglobin in frequency of use as an oxygen transport molecule. Unlike the hemoglobin in red blood cells found in vertebrates, hemocyanins are not bound to blood cells but are instead suspended directly in the hemolymph. Oxygenation causes a color change between the colorless Cu(I) deoxygenated form and the blue Cu(II) oxygenated form.
 W
WHemopexin, also known as beta-1B-glycoprotein, is a glycoprotein that in humans is encoded by the HPX gene and belongs to hemopexin family of proteins. Hemopexin is the plasma protein enjoys the highest binding affinity for heme.
 W
WHemovanadin is a pale green vanabin protein found in the blood cells, called vanadocytes, of ascidians and other organisms. It is one of the few known vanadium-containing proteins. The German chemist Martin Henze first detected vanadium in ascidians in 1911. Unlike hemocyanin and hemoglobin, hemovanadin is not an oxygen carrier.
 W
WHepcidin is a protein that in humans is encoded by the HAMP gene. Hepcidin is a key regulator of the entry of iron into the circulation in mammals.
 W
WHuman serum albumin is the serum albumin found in human blood. It is the most abundant protein in human blood plasma; it constitutes about half of serum protein. It is produced in the liver. It is soluble in water, and it is monomeric.
 W
WKeyhole limpet hemocyanin (KLH) is a large, multisubunit, oxygen-carrying, metalloprotein that is found in the hemolymph of the giant keyhole limpet, Megathura crenulata, a species of keyhole limpet that lives off the coast of California, from Monterey Bay to Isla Asuncion off Baja California.
 W
WA macroglobulin is a plasma globulin of high molecular weight.
 W
WMannose-binding lectin (MBL), also called mannan-binding lectin or mannan-binding protein (MBP), is a lectin that is instrumental in innate immunity as an opsonin and via the lectin pathway.
 W
WSerum albumin, often referred to simply as blood albumin, is an albumin found in vertebrate blood. Human serum albumin is encoded by the ALB gene. Other mammalian forms, such as bovine serum albumin, are chemically similar.
 W
WThe serum amyloid P component (SAP) is the identical serum form of amyloid P component (AP), a 25kDa pentameric protein first identified as the pentagonal constituent of in vivo pathological deposits called "amyloid". APCS is its human gene.
 W
WThyroxine-binding globulin (TBG) is a globulin protein that in humans is encoded by the SERPINA7 gene. TBG binds thyroid hormones in circulation. It is one of three transport proteins (along with transthyretin and serum albumin) responsible for carrying the thyroid hormones thyroxine (T4) and triiodothyronine (T3) in the bloodstream. Of these three proteins, TBG has the highest affinity for T4 and T3 but is present in the lowest concentration relative to transthyretin and albumin, which also bind T3 and T4 in circulation. Despite its low concentration, TBG carries the majority of T4 in the blood plasma. Due to the very low concentration of T4 and T3 in the blood, TBG is rarely more than 25% saturated with its ligand. Unlike transthyretin and albumin, TBG has a single binding site for T4/T3. TBG is synthesized primarily in the liver as a 54-kDa protein. In terms of genomics, TBG is a serpin; however, it has no inhibitory function like many other members of this class of proteins.
 W
WTransthyretin (TTR or TBPA) is a transport protein in the serum and cerebrospinal fluid that carries the thyroid hormone thyroxine (T4) and retinol-binding protein bound to retinol. This is how transthyretin gained its name: transports thyroxine and retinol. The liver secretes transthyretin into the blood, and the choroid plexus secretes TTR into the cerebrospinal fluid.
 W
Wvon Willebrand factor (VWF) is a blood glycoprotein involved in hemostasis. It is deficient and/or defective in von Willebrand disease and is involved in many other diseases, including thrombotic thrombocytopenic purpura, Heyde's syndrome, and possibly hemolytic–uremic syndrome. Increased plasma levels in many cardiovascular, neoplastic, and connective tissue diseases are presumed to arise from adverse changes to the endothelium, and may predict an increased risk of thrombosis.