 W
WCombustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vapourise, but when it does, a flame is a characteristic indicator of the reaction. While the activation energy must be overcome to initiate combustion, the heat from a flame may provide enough energy to make the reaction self-sustaining.
 W
WIn chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (Ea) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term Activation Energy was introduced in 1889 by the Swedish scientist Svante Arrhenius.
 W
WAsh or ashes are the solid remnants of fires. Specifically, ash refers to all non-aqueous, non-gaseous residues that remain after something burns. In analytical chemistry, to analyse the mineral and metal content of chemical samples, ash is the non-gaseous, non-liquid residue after complete combustion.
 W
WA backdraft or backdraught is a rapid or explosive burning of superheated gasses in a fire, caused when oxygen rapidly enters an oxygen-depleted environment; for example, when a window or door to an enclosed space is opened or broken. Backdrafts present a serious threat to firefighters. There is some debate concerning whether backdrafts should be considered a type of flashover.
 W
WThe Chapman–Jouguet condition holds approximately in detonation waves in high explosives. It states that the detonation propagates at a velocity at which the reacting gases just reach sonic velocity as the reaction ceases.
 W
WChemical looping combustion (CLC) is a technological process typically employing a dual fluidized bed system. CLC operated with an interconnected moving bed with a fluidized bed system, has also been employed as a technology process. In CLC, a metal oxide is employed as a bed material providing the oxygen for combustion in the fuel reactor. The reduced metal is then transferred to the second bed and re-oxidized before being reintroduced back to the fuel reactor completing the loop. Fig 1 shows a simplified diagram of the CLC process. Fig 2 shows an example of a dual fluidized bed circulating reactor system and a moving bed-fluidized bed circulating reactor system.
 W
WCombustion and Flame is a monthly peer-reviewed scientific journal published by Elsevier on behalf of the Combustion Institute. It covers fundamental research on combustion science. The editors-in-chief are Fokion Egolfopoulos and Thierry Poinsot.
 W
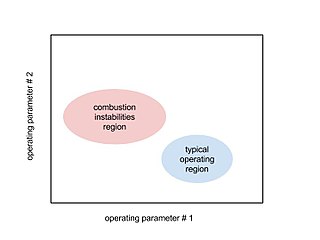
WCombustion instabilities are physical phenomena occurring in a reacting flow in which some perturbations, even very small ones, grow and then become large enough to alter the features of the flow in some particular way.
 W
WThe Combustion Institute is an educational non-profit, international, scientific and engineering society whose purpose is to promote research in combustion science. The institute was established in 1954, and its headquarters are in Pittsburgh, Pennsylvania, United States. The current president of the combustion institute is Philippe Dagaut (2021-).
 W
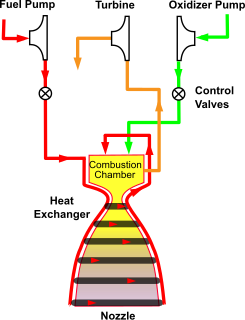
WThe combustion tap-off cycle is a power cycle of a bipropellant rocket engine. The cycle takes a small portion of hot exhaust gas from the rocket engine's combustion chamber and routes it through turbopump turbines to pump fuel before being exhausted. Since fuel is exhausted, the tap-off cycle is considered an open-cycle engine. The cycle is comparable to a gas-generator cycle engine with turbines driven by main combustion chamber exhaust rather than a separate gas generator or preburner.
 W
WA conflagration is a large and destructive fire that threatens human life, animal life, health, and/or property. It may also be described as a blaze or simply a (large) fire. A conflagration can begin accidentally, be naturally caused (wildfire), or intentionally created (arson). A very large fire can produce a firestorm, in which the central column of rising heated air induces strong inward winds, which supply oxygen to the fire. Conflagrations can cause casualties including deaths or injuries from burns, trauma due to collapse of structures and attempts to escape, and smoke inhalation.
 W
WDetonation is a type of combustion involving a supersonic exothermic front accelerating through a medium that eventually drives a shock front propagating directly in front of it. Detonations occur in both conventional solid and liquid explosives, as well as in reactive gases. The velocity of detonation in solid and liquid explosives is much higher than that in gaseous ones, which allows the wave system to be observed with greater detail.
 W
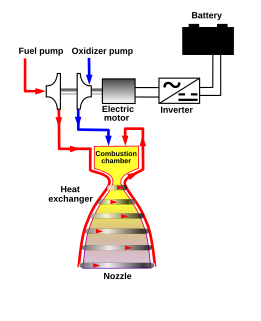
WThe electric-pump-fed engine is a bipropellant rocket engine in which the fuel pumps are electrically powered, and so all of the input propellant is directly burned in the main combustion chamber, and none is diverted to drive the pumps. This differs from traditional rocket engine designs, in which the pumps are driven by a portion of the input propellants.
 W
WExperiments in Fluids is a scientific, peer-reviewed scientific journal published monthly by Springer Science+Business Media. The journal presents contributions that employ existing experimental techniques to gain an understanding of the underlying flow physics in specific areas. These areas include turbulence, aerodynamics, hydrodynamics, convective heat transfer, combustion, turbomachinery, multi-phase flows, and chemical, biological and geological flows. In addition, papers report on investigations combining experimental and analytical/numerical approaches. The journal also publishes letters and review articles.
 W
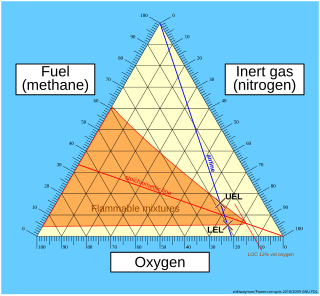
WFlammability diagrams show the control of flammability in mixtures of fuel, oxygen and an inert gas, typically nitrogen. Mixtures of the three gasses are usually depicted in a triangular diagram, known as a ternary plot. Such diagrams are available in the speciality literature. The same information can be depicted in a normal orthogonal diagram, showing only two substances, implicitly using the feature that the sum of all three components is 100 percent. The diagrams below only concerns one fuel; the diagrams can be generalized to mixtures of fuels.
 W
WIn chemistry, the flash point of a volatile material is the lowest temperature at which its vapors ignite if given an ignition source.
 W
WFlue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases from a fireplace, oven, furnace, boiler or steam generator. Quite often, the flue gas refers to the combustion exhaust gas produced at power plants. Its composition depends on what is being burned, but it will usually consist of mostly nitrogen derived from the combustion of air, carbon dioxide, and water vapor as well as excess oxygen. It further contains a small percentage of a number of pollutants, such as particulate matter, carbon monoxide, nitrogen oxides, and sulfur oxides.
 W
WA flue-gas stack, also known as a smoke stack, chimney stack or simply as a stack, is a type of chimney, a vertical pipe, channel or similar structure through which combustion product gases called flue gases are exhausted to the outside air. Flue gases are produced when coal, oil, natural gas, wood or any other fuel is combusted in an industrial furnace, a power plant's steam-generating boiler, or other large combustion device. Flue gas is usually composed of carbon dioxide (CO2) and water vapor as well as nitrogen and excess oxygen remaining from the intake combustion air. It also contains a small percentage of pollutants such as particulate matter, carbon monoxide, nitrogen oxides and sulfur oxides. The flue gas stacks are often quite tall, up to 400 metres (1300 feet) or more, so as to disperse the exhaust pollutants over a greater area and thereby reduce the concentration of the pollutants to the levels required by governmental environmental policy and environmental regulation.
 W
WThe gas-generator cycle is a power cycle of a pumped liquid bipropellant rocket engine. Part of the unburned propellant is burned in a gas generator and the resulting hot gas is used to power the propellant pumps before being exhausted overboard, and lost. Because of this loss, this type of engine is termed open cycle.
 W
WIn mathematics, the Kuramoto–Sivashinsky equation is a fourth-order nonlinear partial differential equation. It is named after Yoshiki Kuramoto and Gregory Sivashinsky, who derived the equation in the late 1970s to model the diffusive instabilities in a laminar flame front. The Kuramoto–Sivashinsky equation is known for its chaotic behavior.
 W
WThe limiting oxygen concentration (LOC), also known as the minimum oxygen concentration (MOC), is defined as the limiting concentration of oxygen below which combustion is not possible, independent of the concentration of fuel. It is expressed in units of volume percent of oxygen. The LOC varies with pressure and temperature. It is also dependent on the type of inert (non-flammable) gas.
 W
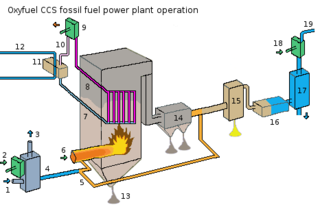
WOxy-fuel combustion is the process of burning a fuel using pure oxygen, or a mixture of oxygen and recirculated flue gas, instead of air. Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatures are possible. Historically, the primary use of oxy-fuel combustion has been in welding and cutting of metals, especially steel, since oxy-fuel allows for higher flame temperatures than can be achieved with an air-fuel flame. It has also received a lot of attention in recent decades as a potential carbon capture and storage technology.
 W
WThe phlogiston theory is a superseded scientific theory that postulated the existence of a fire-like element called phlogiston contained within combustible bodies and released during combustion. The name comes from the Ancient Greek φλογιστόν phlogistón, from φλόξ phlóx (flame). The idea was first proposed in 1667 by Johann Joachim Becher and later put together more formally by Georg Ernst Stahl. Phlogiston theory attempted to explain chemical processes such as combustion and rusting, now collectively known as oxidation. It was challenged by the concomitant weight increase, and was abandoned before the end of the 18th century following experiments by Antoine Lavoisier and others. Phlogiston theory led to experiments which ultimately concluded with the discovery of oxygen.
 W
WA premixed flame is a flame formed under certain conditions during the combustion of a premixed charge of fuel and oxidiser. Since the fuel and oxidiser—the key chemical reactants of combustion—are available throughout a homogeneous stoichiometric premixed charge, the combustion process once initiated sustains itself by way of its own heat release. The majority of the chemical transformation in such a combustion process occurs primarily in a thin interfacial region which separates the unburned and the burned gases. The premixed flame interface propagates through the mixture until the entire charge is depleted. The propagation speed of a premixed flame is known as the flame speed which depends on the convection-diffusion-reaction balance within the flame, i.e. on its inner chemical structure. The premixed flame is characterised as laminar or turbulent depending on the velocity distribution in the unburned pre-mixture.
 W
WA pyrometer is a type of remote-sensing thermometer used to measure the temperature of distant objects. Various forms of pyrometers have historically existed. In the modern usage, it is a device that from a distance determines the temperature of a surface from the amount of the thermal radiation it emits, a process known as pyrometry and sometimes radiometry.
 W
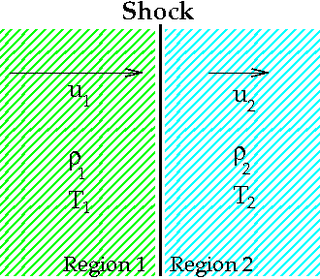
WThe Rankine–Hugoniot conditions, also referred to as Rankine–Hugoniot jump conditions or Rankine–Hugoniot relations, describe the relationship between the states on both sides of a shock wave or a combustion wave in a one-dimensional flow in fluids or a one-dimensional deformation in solids. They are named in recognition of the work carried out by Scottish engineer and physicist William John Macquorn Rankine and French engineer Pierre Henri Hugoniot.
 W
WRollover is a stage of a structure fire when fire gases in a room or other enclosed area ignite. Since heated fire gases, the product of pyrolysis, rise to the ceiling, this is where a rollover phenomenon is most often witnessed. Visually, this may be seen as flames "rolling" across the ceiling, radiating outward from the seat of the fire to the extent of gas spread.
 W
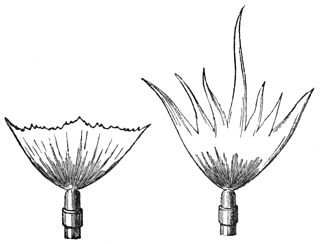
WA sensitive flame is a gas flame which under suitable adjustment of pressure resonates readily with sounds or air vibrations in the vicinity. Noticed by both the American scientist John LeConte and the English physicist William Fletcher Barrett, they recorded the effect that a shrill note had upon a gas flame issuing from a tapering jet. The phenomenon caught the attention of the Irish physicist John Tyndall who gave a lecture on the process to the Royal Institution in January 1867.
 W
WSpontaneous combustion or spontaneous ignition is a type of combustion which occurs by self-heating, followed by thermal runaway and finally, autoignition.
 W
WThe SRM Engine Suite is an engineering software tool used for simulating fuels, combustion and exhaust gas emissions in internal combustion engine applications. It is used worldwide by leading IC engine development organisations and fuel companies. The software is developed, maintained and supported by CMCL Innovations, Cambridge, U.K.
 W
WThe staged combustion cycle is a power cycle of a bipropellant rocket engine. In the staged combustion cycle, propellant flows through multiple combustion chambers, and is thus combusted in stages. The main advantage relative to other rocket engine power cycles is high fuel efficiency, measured through specific impulse, while its main disadvantage is engineering complexity.
 W
WThin filament pyrometry (TFP) is an optical method used to measure temperatures. It involves the placement of a thin filament in a hot gas stream. Radiative emissions from the filament can be correlated with filament temperature. Filaments are typically silicon carbide (SiC) fibers with a diameter of 15 micrometres. Temperatures of about 800–2500 K can be measured.