 W
WAceglutamide, or aceglutamide aluminum, also known as acetylglutamine, is a psychostimulant, nootropic, and antiulcer agent that is marketed in Spain and Japan. It is an acetylated form of the amino acid L-glutamine, the precursor of glutamate in the body and brain. Aceglutamide functions as a prodrug to glutamine with improved potency and stability.
 W
WAcetoxolone is a drug used for peptic ulcer and gastroesophageal reflux disease. It is an acetyl derivative of glycyrrhetinic acid.
 W
WAlicaforsen (Camligo®) is an antisense oligonucleotide therapeutic that targets the messenger RNA for the production of human ICAM-1 protein and is being developed for flares in moderate to severe Inflammatory Bowel Disease (IBD).
 W
WAlka-Seltzer is an effervescent antacid and pain reliever first marketed by the Dr. Miles Medicine Company of Elkhart, Indiana, United States. Alka-Seltzer contains three active ingredients: aspirin (ASA), sodium bicarbonate, and anhydrous citric acid. The aspirin is a pain reliever and anti-inflammatory, the sodium bicarbonate is an antacid, and the citric acid reacts with the sodium bicarbonate and water to form effervescence.
 W
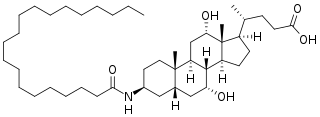
WAramchol is an investigational drug being developed by Galmed Pharmaceuticals as a first-in-class, potentially disease modifying treatment for nonalcoholic steatohepatitis, or NASH, a more advanced condition of non-alcoholic fatty liver disease.
 W
WAxelopran is a drug which is under development by Theravance Biopharma for the treatment of opioid-induced constipation. It acts as a peripherally acting μ-opioid receptor antagonist and also acts on κ-, and δ-opioid receptors, with similar affinity for the μ- and κ-opioid receptors and about an order of magnitude lower affinity for the δ-opioid receptor. Axelopran has potent μ-opioid receptor antagonist activity on the gastrointestinal tract in vivo, and thus it produces a dose-dependent inhibition of opioid-induced delaying in gastric emptying in mice and rats following subcutaneous or oral administration.
 W
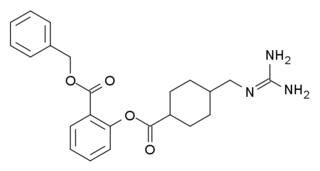
WBenexate (INN) is a drug used in the treatment of acid-related disorders.
 W
WBevenopran is a peripherally acting μ-opioid receptor antagonist that also acts on δ-opioid receptors and was under development by Cubist Pharmaceuticals for the treatment of chronic opioid-induced constipation. It reached phase III clinical trials for this indication before being discontinued.
 W
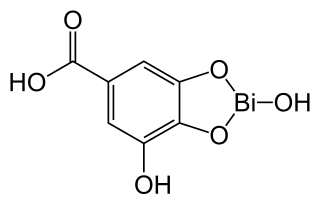
WBismuth subgallate, with a chemical formula C7H5BiO6, is commonly used to treat malodor by deodorizing flatulence and stools. In the United States, it (bismuth subgallate) is the active ingredient in Devrom (internal deodorant), an over-the-counter FDA-approved medicine. Also, it has been used to treat Helicobacter pylori infection and is used in wound therapy. As an internal deodorant, it is commonly used by individuals who have had gastrointestinal stoma surgery, bariatric surgery, fecal incontinence, and irritable bowel syndrome.
 W
WBismuth subsalicylate, sold as generic and under the brand name Pepto-Bismol, is an antacid elixir medication used to treat temporary discomforts of the stomach and gastrointestinal tract, such as nausea, heartburn, indigestion, upset stomach, and diarrhea. It is also commonly known as pink bismuth, but Pepto-Bismol has become a genericized trademark for the substance.
 W
WBromo-Seltzer is a brand of antacid to relieve pain occurring together with heartburn, upset stomach, or acid indigestion.
 W
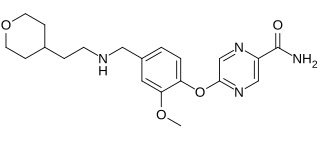
WCamicinal is a motilin agonist for the treatment of gastroparesis.
 W
WCandoxatril is the orally active prodrug of candoxatrilat (UK-73967) human neutral endopeptidase (Neprilysin) as the neutral endopeptidase 24.11 complexed (RB-101) with phosphoramidon degrades and inactivates a number of bioactive peptides. Two multiply connected folding domains of the neutral endopeptidase locus splicing of exons 1, 2a, or 2b to the common exon 3 composed of 24 exons of the human CALLA/NEP gene containing the active site, it is known as peptidase family M13 the gluzincins a faint but significant structural relationship of the metzincins to the thermolysin-like enzymes, Zincin is the simplest descriptor of biological space. The structure reveals two multiply connected folding domains which embrace a large central cavity containing the active site of the 5-indanyl ester prodrug candoxatril and differs from phosphoramidon [N-(N- -L- leucyl)-L-tryptophan] in several respects the structure of human neutral endopeptidase complexed with phosphoramidon is lost due to desolvation of the enzyme and ligand on formation of the complex Candoxatril.
 W
WCarbenoxolone (CBX) is a glycyrrhetinic acid derivative with a steroid-like structure, similar to substances found in the root of the licorice plant. Carbenoxolone is used for the treatment of peptic, esophageal and oral ulceration and inflammation. Electrolyte imbalance is a serious side effect of carbenoxolone when used systemically.
 W
WCetraxate (INN) is an oral gastrointestinal medication which has a cytoprotective effect.
 W
WDrotaverine is an antispasmodic drug, used to enhance cervical dilation during childbirth.
 W
WEcadotril is a neutral endopeptidase inhibitor and determined by the presence of peptidase family M13 as a neutral endopeptidase inhibited by phosphoramidon. Ecadotril is the (S)-enantiomer of racecadotril. NEP-like enzymes include the endothelin-converting enzymes. The peptidase M13 family believed to activate or inactivate oligopeptide (pro)-hormones such as opioid peptides, neprilysin is another member of this group, in the case of the metallopeptidases and aspartic, the nucleophiles clan or family for example MA, is an activated water molecule. The peptidase domain for members of this family also contains a bacterial member and resembles that of thermolysin the predicted active site residues for members of this family and thermolysin occur in the motif HEXXH. Thermolysin complexed with the inhibitor (S)-thiorphan are isomeric thiol-containing inhibitors of endopeptidase EC 24-11.
 W
WAn enema, also known as a clyster, is an injection of fluid into the lower bowel by way of the rectum. Also, the word enema can refer to the liquid so injected, as well as to a device for administering such an injection.
 W
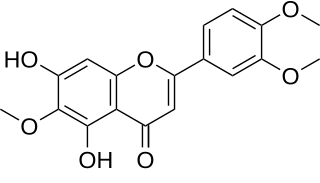
WEupatilin (5,7-Dihydroxy-3',4',6-trimethoxyflavone) is an O-methylated flavone, a type of flavonoids. It can be found in Artemisia asiatica (Asteraceae).
 W
WFerrous tartrate is a chemical compound and the iron(II) salt of tartaric acid.
 W
WLinaclotide, sold under the brand name Linzess in the US and Mexico, and as Constella elsewhere) is a drug used to treat irritable bowel syndrome with constipation and chronic constipation with no known cause. It has a black box warning about the risk of serious dehydration in children in the US; the most common adverse effects in others are gastrointestinal.
 W
WLubiprostone is a medication used in the management of chronic idiopathic constipation, predominantly irritable bowel syndrome-associated constipation in women and opioid-induced constipation. The drug is owned by Mallinckrodt and is marketed by Takeda Pharmaceutical Company.
 W
WMalotilate (INN) is a drug used in the treatment of liver disease. It has been shown to facilitate liver regeneration in rats.
 W
WObeticholic acid, is a semi-synthetic bile acid analogue which has the chemical structure 6α-ethyl-chenodeoxycholic acid. It is used as a drug to treat primary biliary cholangitis, and is undergoing development for several other liver diseases and related disorders. Intercept Pharmaceuticals Inc. hold the worldwide rights to develop OCA outside Japan and China, where it is licensed to Dainippon Sumitomo Pharma.
 W
WPegvaliase, sold under the brand name Palynziq, is a medication for the treatment of the genetic disease phenylketonuria. Chemically, it is a pegylated derivative of the enzyme phenylalanine ammonia-lyase that metabolizes phenylalanine to reduce its blood levels.
 W
WRelamorelin is a synthetic peptide, centrally penetrant, selective agonist of the ghrelin/growth hormone secretagogue receptor (GHSR) which is under development by Allergan pharmaceuticals for the treatment of diabetic gastroparesis, chronic idiopathic constipation, and anorexia nervosa. It is a pentapeptide and an analogue of ghrelin with improved potency and pharmacokinetics. In humans, relamorelin produces increases in plasma growth hormone, prolactin, and cortisol levels, and, like other GHSR agonists, increases appetite. As of June 2015, relamorelin is in phase II clinical trials for diabetic gastroparesis and constipation. The United States Food and Drug Administration (FDA) has granted Fast Track designation to relamorelin for diabetic gastroparesis.
 W
WRolaids is an American brand of calcium and magnesium-based antacid produced by Chattem. It was invented by American chemist Irvine W. Grote in the late 1920s, and originated with manufacturing in Chattanooga, Tennessee under one of Chattem's forerunner companies, which manufactured the brand for Warner-Lambert; Warner-Lambert merged with Pfizer in 2000.
 W
WSeidlitz powders is the generic name under which a commonly known laxative and digestion regulator was marketed and sold by numerous manufacturers under names such as "Rexall Seidlitz Powders", particularly in the late 19th and early 20th centuries.
 W
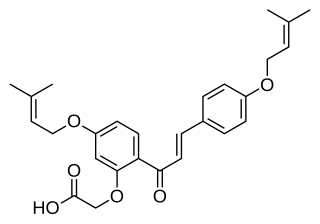
WSofalcone (INN) is an oral gastrointestinal medication used in Japan. It is a synthetic analog of sophoradin, a type of natural phenol found in Sophora tonkinensis, an herb used in traditional Chinese medicine.
 W
WSucralfate, sold under various brand names, is a medication used to treat stomach ulcers, gastroesophageal reflux disease (GERD), radiation proctitis, and stomach inflammation and to prevent stress ulcers. Its usefulness in people infected by H. pylori is limited. It is used by mouth and rectally.
 W
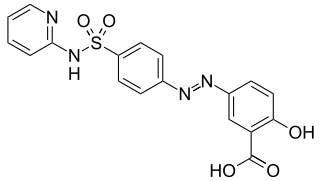
WSulfasalazine (SSZ), sold under the trade name Azulfidine among others, is a medication used to treat rheumatoid arthritis, ulcerative colitis, and Crohn's disease. It is considered by some to be a first line treatment in rheumatoid arthritis. It is taken by mouth.
 W
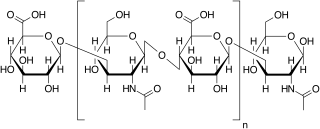
WSulglicotide is a drug used for peptic ulcer and gastro-oesophageal reflux disease.
 W
WTeduglutide is a 33-membered polypeptide and glucagon-like peptide-2 (GLP-2) analog that is used for the treatment of short bowel syndrome. It works by promoting mucosal growth and possibly restoring gastric emptying and secretion. In Europe it has been granted orphan drug status and is marketed under the brand Revestive by Nycomed. It was approved by the United States under the name Gattex on 21 December 2012 and also is an orphan drug there.
 W
WTenapanor, used in form of tenapanor hydrochloride and sold under the brand name Ibsrela, is a treatment for adults with a disease of the gut called irritable bowel syndrome with constipation commonly referred to as IBS-C.
 W
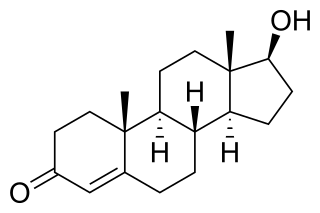
WTestosterone (T) is a medication and naturally occurring steroid hormone. It is used to treat male hypogonadism, gender dysphoria, and certain types of breast cancer. It may also be used to increase athletic ability in the form of doping. It is unclear if the use of testosterone for low levels due to aging is beneficial or harmful. Testosterone can be used as a gel or patch that is applied to the skin, injection into a muscle, tablet that is placed in the cheek, or tablet that is taken by mouth.
 W
WTidiacic is a hepatoprotective drug. It is a component of tidiacic arginine.
 W
WTiropramide (INN) is an antispasmodic.
 W
WUlimorelin is a drug with a modified cyclic peptide structure which acts as a selective agonist of the ghrelin/growth hormone secretagogue receptor (GHSR-1a).. Unlike many related drugs, ulimorelin has little or no effect on growth hormone (GH) release in rats. However, like ghrelin and other ghrelin agonists, ulimorelin does stimulate GH release with concomitant increases in insulin-like growth factor 1 (IGF-1) in humans. It has been researched for enhancing gastrointestinal motility, especially in gastroparesis and in aiding recovery of bowel function following gastrointestinal surgery, where opioid analgesic drugs used for post-operative pain relief may worsen existing constipation. While ulimorelin has been shown to increase both upper and lower gastrointestinal motility in rats, and showed promising results initially in humans, it failed in pivotal clinical trials in post operative ileus.
 W
WUrsodeoxycholic acid (UDCA), also known as ursodiol, is a secondary bile acid, produced in humans and most other species from metabolism by intestinal bacteria. It is synthesized in the liver in some species, and was first identified in bear bile, which is the derivation of its name Ursus. In purified form, it has been used to treat or prevent several diseases of the liver or bile ducts.
 W
WVolixibat is a medication under development as a possible treatment for nonalcoholic steatohepatitis (NASH), the most severe form of non-alcoholic fatty liver disease (NAFLD). No other pharmacotherapy yet exists for NASH, so there is interest in whether volixibat can prove to be both safe and effective. To encourage development and testing, the U.S. Food and Drug Administration (FDA) has issued fast track status.
 W
WVonoprazan is a first-in-class potassium-competitive acid blocker. It was approved in the Japanese market in February 2015.