 W
WWater quality refers to the chemical, physical, and biological characteristics of water based on the standards of its usage. It is most frequently used by reference to a set of standards against which compliance, generally achieved through treatment of the water, can be assessed. The most common standards used to monitor and assess water quality convey the health of ecosystems, safety of human contact, and condition of drinking water. Water quality has a significant impact on water supply and oftentimes determines supply options.
 W
WAn algal bloom or algae bloom is a rapid increase or accumulation in the population of algae in freshwater or marine water systems, and is often recognized by the discoloration in the water from their pigments. The term algae encompasses many types of aquatic photosynthetic organisms, both macroscopic, multicellular organisms like seaweed and microscopic, unicellular organisms like cyanobacteria. Algal bloom commonly refers to rapid growth of microscopic, unicellular algae, not macroscopic algae. An example of a macroscopic algal bloom is a kelp forest.
 W
WOceanic anoxic events or anoxic events (anoxia conditions) were intervals in the Earth's past where portions of oceans became depleted in oxygen (O2) over a large geographic areas. During some of these events, euxinia, waters that contain hydrogen sulfide, H2S, developed. Although anoxic events have not happened for millions of years, the geological record shows that they happened many times in the past. Anoxic events coincided with several mass extinctions and may have contributed to them. These mass extinctions include some that geobiologists use as time markers in biostratigraphic dating. Many geologists believe oceanic anoxic events are strongly linked to slowing of ocean circulation, climatic warming, and elevated levels of greenhouse gases. Researchers have proposed enhanced volcanism (the release of CO2) as the "central external trigger for euxinia".
 W
WBacteriological water analysis is a method of analysing water to estimate the numbers of bacteria present and, if needed, to find out what sort of bacteria they are. It represents one aspect of water quality. It is a microbiological analytical procedure which uses samples of water and from these samples determines the concentration of bacteria. It is then possible to draw inferences about the suitability of the water for use from these concentrations. This process is used, for example, to routinely confirm that water is safe for human consumption or that bathing and recreational waters are safe to use.
 W
WBiochemical oxygen demand (BOD) is the amount of dissolved oxygen needed by aerobic biological organisms to break down organic material present in a given water sample at certain temperature over a specific time period. The BOD value is most commonly expressed in milligrams of oxygen consumed per litre of sample during 5 days of incubation at 20 °C and is often used as a surrogate of the degree of organic pollution of water.
 W
WA bioindicator is any species or group of species whose function, population, or status can reveal the qualitative status of the environment. For example, copepods and other small water crustaceans that are present in many water bodies can be monitored for changes that may indicate a problem within their ecosystem. Bioindicators can tell us about the cumulative effects of different pollutants in the ecosystem and about how long a problem may have been present, which physical and chemical testing cannot.
 W
WColiform bacteria are defined as Rod shaped Gram-negative non-spore forming and motile or non-motile bacteria which can ferment lactose with the production of acid and gas when incubated at 35–37°C. Due to the limited ability of certain coliform bacteria to ferment lactose, the definition has changed to bacteria containing the enzyme β-galactosidase. They are a commonly used indicator of sanitary quality of foods and water. Coliforms can be found in the aquatic environment, in soil and on vegetation; they are universally present in large numbers in the feces of warm-blooded animals. While coliforms themselves are not normally causes of serious illness, they are easy to culture, and their presence is used to indicate that other pathogenic organisms of fecal origin may be present. Such pathogens include disease-causing bacteria, viruses, or protozoa and many multicellular parasites. Coliform procedures are performed in aerobic or anaerobic conditions.
 W
WThe color of water varies with the ambient conditions in which that water is present. While relatively small quantities of water appear to be colorless, pure water has a slight blue color that becomes a deeper blue as the thickness of the observed sample increases. The blue hue of water is an intrinsic property and is caused by selective absorption and scattering of white light. Dissolved elements or suspended impurities may give water a different color.
 W
WColored dissolved organic matter (CDOM) is the optically measurable component of dissolved organic matter in water. Also known as chromophoric dissolved organic matter, yellow substance, and gelbstoff, CDOM occurs naturally in aquatic environments and is a complex mixture of many hundreds to thousands of individual, unique organic matter molecules, which are primarily leached from decaying detritus and organic matter. CDOM most strongly absorbs short wavelength light ranging from blue to ultraviolet, whereas pure water absorbs longer wavelength red light. Therefore, water with little or no CDOM, such as the open ocean, appears blue. Waters containing high amounts of CDOM can range from brown, as in many rivers, to yellow and yellow-brown in coastal waters. In general, CDOM concentrations are much higher in fresh waters and estuaries than in the open ocean, though concentrations are highly variable, as is the estimated contribution of CDOM to the total dissolved organic matter pool.
 W
WDissolved organic carbon (DOC) is the fraction of organic carbon operationally defined as that which can pass through a filter with a pore size typically between 0.22 and 0.7 micrometers. The fraction remaining on the filter is called particulate organic carbon (POC).
 W
WFormazine (formazin) is a heterocyclic polymer produced by reaction of hexamethylenetetramine with hydrazine sulfate.
 W
WA harmful algal bloom (HAB) contains organisms that can severely lower oxygen levels in natural waters, killing marine life. Some HABs are associated with algae-produced toxins. Blooms can last from a few days to many months. After the bloom dies, the microbes which decompose the dead algae use up even more of the oxygen, which can create fish die-offs. When these zones of depleted oxygen cover a large area for an extended period of time, they are referred to as dead zones, where neither fish nor plants are able to survive.
 W
WHypoxia refers to low oxygen conditions. Normally, 20.9% of the gas in the atmosphere is oxygen. The partial pressure of oxygen in the atmosphere is 20.9% of the total barometric pressure. In water, oxygen levels are much lower, approximately 7 ppm 0.0007% in good quality water, and fluctuate locally depending on the presence of photosynthetic organisms and relative distance to the surface.
 W
WMicrocystins—or cyanoginosins—are a class of toxins produced by certain freshwater blue-green algae. Over 50 different microcystins have been discovered so far, of which microcystin-LR is the most common. Chemically they are cyclic heptapeptides produced through nonribosomal peptide synthases.
 W
WNitrate is a polyatomic ion with the chemical formula NO−3. Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all nitrates are soluble in water. A common example of an inorganic nitrate salt is potassium nitrate. Removal of one electron yields the nitrate radical, also called nitrogen trioxide NO3.
 W
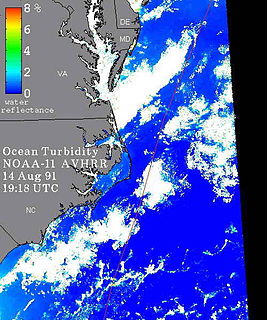
WOcean turbidity is a measure of the amount of cloudiness or haziness in sea water caused by individual particles that are too small to be seen without magnification. Highly turbid ocean waters are those with many scattering particulates in them. In both highly absorbing and highly scattering waters, visibility into the water is reduced. The highly scattering (turbid) water still reflects much light while the highly absorbing water, such as a blackwater river or lake, is very dark. The scattering particles that cause the water to be turbid can be composed of many things, including sediments and phytoplankton.
 W
WOxygen saturation is a relative measure of the concentration of oxygen that is dissolved or carried in a given medium as a proportion of the maximal concentration that can be dissolved in that medium. It can be measured with a dissolved oxygen probe such as an oxygen sensor or an optode in liquid media, usually water. The standard unit of oxygen saturation is percent (%).
 W
WOxygen saturation is the fraction of oxygen-saturated hemoglobin relative to total hemoglobin in the blood. The human body requires and regulates a very precise and specific balance of oxygen in the blood. Normal arterial blood oxygen saturation levels in humans are 95–100 percent. If the level is below 90 percent, it is considered low and called hypoxemia. Arterial blood oxygen levels below 80 percent may compromise organ function, such as the brain and heart, and should be promptly addressed. Continued low oxygen levels may lead to respiratory or cardiac arrest. Oxygen therapy may be used to assist in raising blood oxygen levels. Oxygenation occurs when oxygen molecules enter the tissues of the body. For example, blood is oxygenated in the lungs, where oxygen molecules travel from the air and into the blood. Oxygenation is commonly used to refer to medical oxygen saturation.
 W
WIn chemistry, pH (, denoting 'potential of hydrogen' or 'power of hydrogen') is a scale used to specify the acidity or basicity of an aqueous solution. Acidic solutions (solutions with higher concentrations of H+ ions) are measured to have lower pH values than basic or alkaline solutions.
 W
WSalinity is the saltiness or amount of salt dissolved in a body of water, called saline water. This is usually measured in . Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological processes within it, and is a thermodynamic state variable that, along with temperature and pressure, governs physical characteristics like the density and heat capacity of the water.
 W
WSettling is the process by which particulates settle to the bottom of a liquid and form a sediment. Particles that experience a force, either due to gravity or due to centrifugal motion will tend to move in a uniform manner in the direction exerted by that force. For gravity settling, this means that the particles will tend to fall to the bottom of the vessel, forming a slurry at the vessel base.
 W
WSPEARpesticides is a trait based biological indicator system for streams which quantitatively links pesticide contamination to the composition of invertebrate communities. The approach uses species traits that characterize the ecological requirements posed by pesticide contamination in running waters. Therefore, it is highly specific and only slightly influenced by other environmental factors. SPEARpesticides is linked to the quality classes of the EU Water Framework Directive (WFD)
 W
WTotal carbon (TC) is an analytical measurement for carbon content. This measurement is commonly found in environmental and pharmaceutical analysis.
 W
WTotal dissolved solids (TDS) is a measure of the dissolved combined content of all inorganic and organic substances present in a liquid in molecular, ionized, or micro-granular suspended form. TDS concentrations are often reported in parts per million (ppm). Water TDS concentrations can be determined using a digital meter.
 W
WTotal organic carbon (TOC) is the amount of carbon found in an organic compound and is often used as a non-specific indicator of water quality or cleanliness of pharmaceutical manufacturing equipment. TOC may also refer to the amount of organic carbon in soil, or in a geological formation, particularly the source rock for a petroleum play; 2% is a rough minimum. For marine surface sediments average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins.
 W
WTurbidity is the cloudiness or haziness of a fluid caused by large numbers of individual particles that are generally invisible to the naked eye, similar to smoke in air. The measurement of turbidity is a key test of water quality.
 W
WWastewater quality indicators are laboratory test methodologies to assess suitability of wastewater for disposal or re-use. Tests selected and desired test results vary with the intended use or discharge location. Tests measure physical, chemical, and biological characteristics of the waste water.