 W
WIn oceanic biogeochemistry, the continental shelf pump is proposed to operate in the shallow waters of the continental shelves, acting as a mechanism to transport carbon from surface waters to the interior of the adjacent deep ocean.
 W
WEcology is a branch of biology concerning the spatial and temporal patterns of the distribution and abundance of organisms, including the causes and consequences. Topics of interest include the biodiversity, distribution, biomass, and populations of organisms, as well as cooperation and competition within and between species. Ecosystems are dynamically interacting systems of organisms, the communities they make up, and the non-living components of their environment. Ecosystem processes, such as primary production, pedogenesis, nutrient cycling, and niche construction, regulate the flux of energy and matter through an environment. These processes are sustained by organisms with specific life history traits.
 W
WIn oceanic biogeochemistry, the f-ratio is the fraction of total primary production fuelled by nitrate. The ratio was originally defined by Richard Eppley and Bruce Peterson in one of the first papers estimating global oceanic production. This fraction was originally believed significant because it appeared to directly relate to the sinking (export) flux of organic marine snow from the surface ocean by the biological pump. However, this interpretation relied on the assumption of a strong depth-partitioning of a parallel process, nitrification, that more recent measurements has questioned.
 W
WThe Gaia Paradigm, also known as the Gaia theory or the Gaia principle, proposes that living organisms interact with their inorganic surroundings on Earth to form a synergistic and self-regulating, complex system that helps to maintain and perpetuate the conditions for life on the planet.
 W
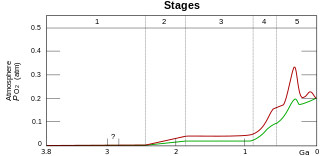
WBefore photosynthesis evolved, Earth's atmosphere had no free oxygen (O2). Photosynthetic prokaryotic organisms that produced O2 as a waste product lived long before the first build-up of free oxygen in the atmosphere, perhaps as early as 3.5 billion years ago. The oxygen they produced would have been rapidly removed from the oceans by weathering of reducing minerals, most notably iron. This rusting led to the deposition of iron oxide on the ocean floor, forming banded iron formations. Thus, the oceans rusted and turned red. Oxygen only began to persist in the atmosphere in small quantities about 50 million years before the start of the Great Oxygenation Event. This mass oxygenation of the atmosphere resulted in rapid buildup of free oxygen. At current rates of primary production, today's concentration of oxygen could be produced by photosynthetic organisms in 2,000 years. In the absence of plants, the rate of oxygen production by photosynthesis was slower in the Precambrian, and the concentrations of O2 attained were less than 10% of today's and probably fluctuated greatly; oxygen may even have disappeared from the atmosphere again around 1.9 billion years ago. These fluctuations in oxygen concentration had little direct effect on life, with mass extinctions not observed until the appearance of complex life around the start of the Cambrian period, 541 million years ago. The presence of O2 provided life with new opportunities. Aerobic metabolism is more efficient than anaerobic pathways, and the presence of oxygen created new possibilities for life to explore. Since the start of the Cambrian period, atmospheric oxygen concentrations have fluctuated between 15% and 35% of atmospheric volume. The maximum of 35% was reached towards the end of the Carboniferous period (about 300 million years ago), a peak which may have contributed to the large size of insects and amphibians at that time. Whilst human activities, such as the burning of fossil fuels, affect relative carbon dioxide concentrations, their effect on the much larger concentration of oxygen is less significant.
 W
WEville Gorham was a Canadian-American scientist whose focus has been understanding the chemistry of fresh waters and the ecology and biogeochemistry of peatlands. In the process, Gorham made a number of practical contributions that included discovering the influence of acid rain in lake acidification, plus the importance of the biological magnification of radioactive fallout isotopes in northern food chains. The former led to legislation and redesign of the power plants of the world to scrub sulfur, and the latter was an early step toward the establishment of an atmospheric nuclear test ban treaty.
 W
WJelly-falls are marine carbon cycling events whereby gelatinous zooplankton, primarily cnidarians, sink to the seafloor and enhance carbon and nitrogen fluxes via rapidly sinking particulate organic matter. These events provide nutrition to benthic megafauna and bacteria. Jelly-falls have been implicated as a major “gelatinous pathway” for the sequestration of labile biogenic carbon through the biological pump. These events are common in protected areas with high levels of primary production and water quality suitable to support cnidarian species. These areas include estuaries and several studies have been conducted in fjords of Norway.
 W
WThe Max Planck Institute for Biogeochemistry is located in Jena, Germany. It was created in 1997, and moved into new buildings 2002. It is one of 80 institute in the Max Planck Society.
 W
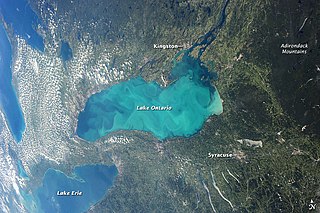
WA whiting event is a phenomenon that occurs when a suspended cloud of fine-grained calcium carbonate precipitates in water bodies, typically during summer months, as a result of photosynthetic microbiological activity or sediment disturbance. The phenomenon gets its name from the white, chalky color it imbues to the water. These events have been shown to occur in temperate waters as well as tropical ones, and they can span for hundreds of meters. They can also occur in both marine and freshwater environments. The origin of whiting events is debated among the scientific community, and it is unclear if there is a single, specific cause. Generally, they are thought to result from either bottom sediment re-suspension or by increased activity of certain microscopic life such as phytoplankton. Because whiting events affect aquatic chemistry, physical properties, and carbon cycling, studying the mechanisms behind them holds scientific relevance in various ways.