 W
WChromium carbonyl, also known as chromium hexacarbonyl, is the chemical compound with the formula Cr(CO)6. At room temperature the solid is stable to air, although it does have a high vapor pressure and sublimes readily. Cr(CO)6 is zerovalent, meaning that Cr has an oxidation state of zero, and it is a homoleptic complex, which means that all the ligands are identical. The complex is octahedral with Cr–C and C–O distances of 1.91 and 1.14 Å, respectively.
 W
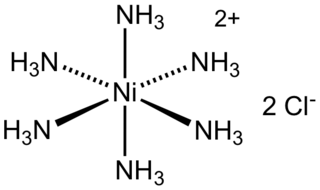
WHexaamminenickel chloride is the chemical compound with the formula [Ni(NH3)6]Cl2. It is the chloride salt of the metal ammine complex [Ni(NH3)6]2+. The cation features six ammonia (called ammines in coordination chemistry) ligands attached to the nickel(II) ion.
 W
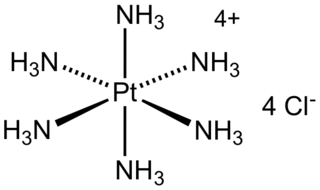
WHexaammineplatinum(IV) chloride is the chemical compound with the formula [Pt(NH3)6]Cl4. It is the chloride salt of the metal ammine complex [Pt(NH3)6]4+. The cation features six ammonia (called ammines in coordination chemistry) ligands attached to the platinum(IV) ion. It is a white, water soluble solid.
 W
WHexaamminecobalt(III) chloride is the chemical compound with the formula [Co(NH3)6]Cl3. It is the chloride salt of the coordination complex [Co(NH3)6]3+, which is considered an archetypal "Werner complex", named after the pioneer of coordination chemistry, Alfred Werner. The cation itself is a metal ammine complex with six ammonia ligands attached to the cobalt(III) ion.
 W
WIridium hexafluoride, also iridium(VI) fluoride, (IrF6) is a compound of iridium and fluorine and one of the seventeen known binary hexafluorides. It is one of only a few compounds with iridium in the oxidation state +6.
 W
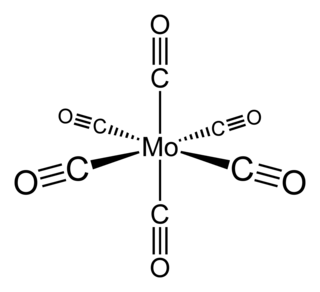
WMolybdenum hexacarbonyl (also called molybdenum carbonyl) is the chemical compound with the formula Mo(CO)6. This colorless solid, like its chromium and tungsten analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero oxidation state.
 W
WMolybdenum hexafluoride, also molybdenum(VI) fluoride, is the inorganic compound with the formula MoF6. It is the highest fluoride of molybdenum. A colourless solid, it melts just below room temperature and boils in 34 °C. It is one of the seventeen known binary hexafluorides.
 W
WNeptunium(VI) fluoride (NpF6) is the highest fluoride of neptunium, it is also one of seventeen known binary hexafluorides. It is an orange volatile crystalline solid. It is relatively hard to handle, being very corrosive, volatile and radioactive. Neptunium hexafluoride is stable in dry air but reacts vigorously with water.
 W
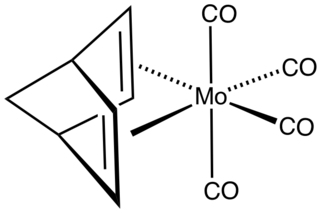
W(Norbornadiene)molybdenum tetracarbonyl is the organomolybdenum compound with the formula (C7H9)Mo(CO)4. Structurally, the compound consists of the norbornadiene bonded to a Mo(CO)4 fragment. The compound is a yellow, volatile solid. It is prepared by thermal or photochemical substitution of molybdenum hexacarbonyl. The compound was originally examined as a potential antiknock agent.
 W
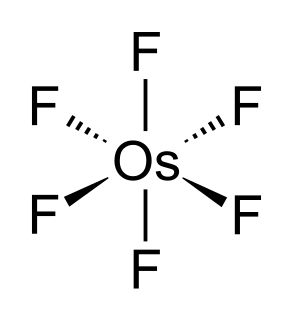
WOsmium hexafluoride, also osmium(VI) fluoride, (OsF6) is a compound of osmium and fluorine, and one of the seventeen known binary hexafluorides.
 W
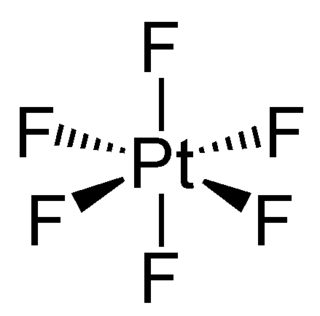
WPlatinum hexafluoride is the chemical compound with the formula PtF6, and is one of seventeen known binary hexafluorides. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation state. With only four d-electrons, it is paramagnetic with a triplet ground state. PtF6 is a strong fluorinating agent and one of the strongest oxidants, capable of oxidising xenon and O2. PtF6 is octahedral in both the solid state and in the gaseous state. The Pt-F bond lengths are 185 picometers.
 W
WPlutonium hexafluoride is the highest fluoride of plutonium, and is of interest for laser enrichment of plutonium, in particular for the production of pure plutonium-239 from irradiated uranium. This pure plutonium is needed to avoid premature ignition of low-mass nuclear weapon designs by neutrons produced by spontaneous fission of plutonium-240.
 W
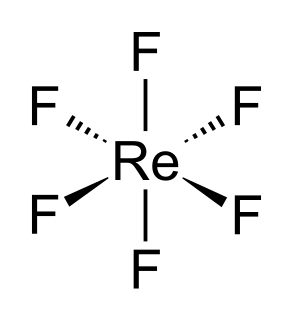
WRhenium hexafluoride, also rhenium(VI) fluoride, (ReF6) is a compound of rhenium and fluorine and one of the seventeen known binary hexafluorides.
 W
WRhodium hexafluoride, also rhodium(VI) fluoride, (RhF6) is the inorganic compound of rhodium and fluorine. A black volatile solid, it is a highly reactive material, and a rare example of a rhodium(VI) compound. It is one of seventeen known binary hexafluoride.
 W
WSulfur hexafluoride (SF6) or sulphur hexafluoride (British spelling) is an extremely potent and persistent greenhouse gas that is primarily utilized as an electrical insulator and arc suppressant. It is inorganic, colorless, odorless, non-flammable, and non-toxic. SF6 has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. It is a hypervalent molecule.
 W
WTechnetium hexafluoride or technetium(VI) fluoride (TcF6) is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. The other such compound is technetium(VI) chloride, TcCl6. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7. Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium.
 W
WTungsten hexacarbonyl (also called tungsten carbonyl) is the chemical compound with the formula W(CO)6. This complex gave rise to the first example of a dihydrogen complex.
 W
WTungsten hexachloride is the chemical compound of tungsten and chlorine with the formula WCl6. This dark violet blue species exists as a volatile solid under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. Other examples of charge-neutral hexachlorides are ReCl6 and MoCl6. The highly volatile WF6 is also known.
 W
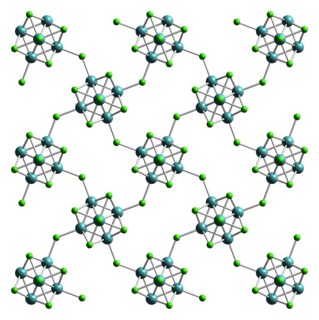
WTungsten(II) chloride is the inorganic compound with the formula W6Cl12. It is a polymeric cluster compound. The material dissolves in concentrated hydrochloric acid, forming (H3O)2[W6Cl14](H2O)x. Heating this salt gives yellow-brown W6Cl12. The structural chemistry resembles that observed for molybdenum(II) chloride.
 W
WTungsten(III) chloride is the inorganic compound with the formula W6Cl18. It is a cluster compound. It is a brown solid, obtainable by chlorination of tungsten(II) chloride. Featuring twelve doubly bridging chloride ligands, the cluster adopts a structure related to the corresponding chlorides of niobium and tantalum. In contrast, W6Cl12 features eight triply bridging chlorides.
 W
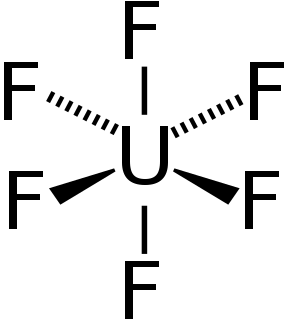
WUranium hexafluoride (UF6), colloquially known as "hex" in the nuclear industry, is a compound used in the process of enriching uranium, which produces fuel for nuclear reactors and nuclear weapons.