 W
WPsychedelics are a class of hallucinogenic drugs whose primary effect is to trigger non-ordinary states of consciousness via serotonin 2A receptor agonism. This causes specific psychological, visual and auditory changes, and often a substantially altered state of consciousness. The "classical" psychedelics, the psychedelics with the largest scientific and cultural influence, are mescaline, LSD, psilocybin, and DMT.
 W
W2CBCB-NBOMe (NBOMe-TCB-2) is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2007 at Purdue University as part of the ongoing research program of the team led by David Nichols focusing on the mapping of the specific amino acid residues responsible for ligand binding to the 5HT2A receptor. 2CBCB-NBOMe acts as a potent and selective agonist for the 5-HT2A and 5-HT2C receptors, with a Ki of 0.27nM at the human 5-HT2A receptor, a similar potency to other agonists such as TCB-2, NBOMe-2C-I and Bromo-DragonFLY.
 W
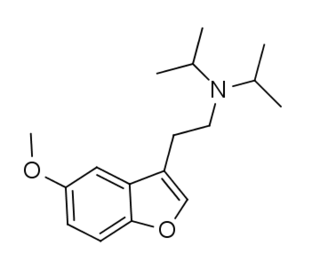
W5-MeO-DiBF is a psychedelic that has been sold online as a designer drug and was first definitively identified in December 2015 by a forensic laboratory in Slovenia. It is thought to act as an agonist for the 5-HT1A and 5-HT2 family of serotonin receptors. It is related in structure to the psychedelic tryptamine derivative 5-MeO-DiPT, but with the indole nitrogen replaced by oxygen, making 5-MeO-DiBF a benzofuran derivative. It is several times less potent as a serotonin agonist than 5-MeO-DiPT and with relatively more activity at 5-HT1A, but still shows strongest effects at the 5-HT2 family of receptors.
 W
WALD-52, also known as 1-acetyl-LSD, is a chemical analogue of lysergic acid diethylamide (LSD). It was originally discovered by Albert Hofmann in 1957 but was not widely studied until the rise in popularity of psychedelics in the 1960s.
 W
WALPHA (alpha-ethyl-3,4-methylenedioxybenzylamine) is a lesser-known psychedelic drug and a substituted benzylamine. It is also the benzylamine analog of 3,4-methylenedioxyamphetamine (MDA). ALPHA was first synthesized by Alexander Shulgin. In his book PIHKAL on the MDA page, the threshold dosage is listed as 10 mg. At mild threshold dosages there are eyes-closed "dreams" with some body tingling, at higher doses was reported to produce a pleasant, positive feeling. This compound is not anoretic at any dose. It lasts about 3 hours. Very little data exists about the pharmacological properties, metabolism, and toxicity of ALPHA.
 W
WClub drugs, also called rave drugs, or party drugs are a loosely defined category of recreational drugs which are associated with discothèques in the 1970s and nightclubs, dance clubs, electronic dance music (EDM) parties, and raves in the 1980s to today. Unlike many other categories, such as opiates and benzodiazepines, which are established according to pharmaceutical or chemical properties, club drugs are a "category of convenience", in which drugs are included due to the locations they are consumed and/or where the user goes while under the influence of the drugs. Club drugs are generally used by adolescents and young adults. This group of drugs is also called "designer drugs", as most are synthesized in a chemical lab rather than being sourced from plants or opiates.
 W
WCohoba is a Taíno Indian transliteration for a ceremony in which the ground seeds of the cojóbana tree were inhaled, the Y-shaped nasal snuff tube used to inhale the substance, and the psychoactive drug that was inhaled. Use of this substance produced a hallucinogenic, entheogenic, or psychedelic effect. The cojóbana tree is believed by some to be Anadenanthera peregrina although it may have been a generalized term for psychotropics, including the quite toxic Datura and related genera (Solanaceae). The corresponding ceremony using cohoba-laced tobacco is transliterated as cojibá. This was said to have produced the sense of a visionary journey of the kind associated with the practice of shamanism.
 W
WN,N-Dimethyltryptamine is a substituted tryptamine that occurs in many plants and animals and which is both a derivative and a structural analog of tryptamine. It is used as a recreational psychedelic drug and prepared by various cultures for ritual purposes as an entheogen.
 W
WN,N-Dimethyltryptamine is a substituted tryptamine that occurs in many plants and animals and which is both a derivative and a structural analog of tryptamine. It is used as a recreational psychedelic drug and prepared by various cultures for ritual purposes as an entheogen.
 W
WEfavirenz (EFV), sold under the brand names Sustiva among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. It is sold both by itself and in combination as efavirenz/emtricitabine/tenofovir. It is taken by mouth.
 W
WEnadoline is a drug which acts as a highly selective κ-opioid agonist.
 W
Wpara-Fluorophenylpiperazine is a piperazine derivative with mildly psychedelic and euphoriant effects. It has been sold as an ingredient in legal recreational drugs known as "Party pills", initially in New Zealand and subsequently in other countries around the world.
 W
WIbotenic acid or (S)-2-amino-2-(3-hydroxyisoxazol-5-yl)acetic acid, also referred to as ibotenate, is a chemical compound and psychoactive drug which occurs naturally in Amanita muscaria and related species of mushrooms typically found in the temperate and boreal regions of the northern hemisphere. It is a conformationally-restricted analogue of the neurotransmitter glutamate, and due to its structural similarity to this neurotransmitter, acts as a non-selective glutamate receptor agonist. Because of this, ibotenic acid can be a powerful neurotoxin, and is employed as a "brain-lesioning agent" through cranial injections in scientific research.
 W
WInhalants are a broad range of household and industrial chemicals whose volatile vapors or pressurized gases can be concentrated and breathed in via the nose or mouth to produce intoxication, in a manner not intended by the manufacturer. They are inhaled at room temperature through volatilization or from a pressurized container, and do not include drugs that are sniffed after burning or heating. For example, amyl nitrite (poppers), nitrous oxide and toluene – a solvent widely used in contact cement, permanent markers, and certain types of glue – are considered inhalants, but smoking tobacco, cannabis, and crack are not, even though these drugs are inhaled as smoke or vapor.
 W
W6-Isopropyl-6-nor-lysergic acid diethylamide (IP-LAD) is an analog of lysergic acid diethylamide (LSD) developed by the team of David E. Nichols. In studies on mice, it was found to be approximately 40% the potency of LSD, compared to the 60% increase in potency seen with ETH-LAD and roughly equivalent potency in AL-LAD and PRO-LAD.
 W
WLorcaserin, marketed under the brand name Belviq is a weight-loss drug developed by Arena Pharmaceuticals. It reduces appetite by activating a type of serotonin receptor known as the 5-HT2C receptor in a region of the brain called the hypothalamus, which is known to control appetite. It was removed from the market in the United States in 2020 due to an increased risk of cancer detected in users of Belviq.
 W
W5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine) or O-methyl-bufotenin is a psychedelic of the tryptamine class. It is found in a wide variety of plant species, and also is secreted by the glands of at least one toad species, the Sonoran Desert toad. Like its close relatives DMT and bufotenin (5-HO-DMT), it has been used as an entheogen in South America. Slang terms include Five-methoxy, The power, and Toad venom.
 W
W1-Methylamino-1-(3,4-methylenedioxyphenyl)propane or M-ALPHA is an empathogen, reported by Alexander Shulgin in his book PIHKAL as a positional isomer of MDMA, and subsequently found being sold as a designer drug in the UK in 2010, and reported to the EMCDDA new drug monitoring service. It was described by Alexander Shulgin as similar in action to its demethylated homologue, ALPHA, but with roughly twice the duration and twice the potency.
 W
W3,4-Methylenedioxyamphetamine is an empathogen-entactogen, psychostimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In terms of pharmacology, MDA acts most importantly as a serotonin-norepinephrine-dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.
 W
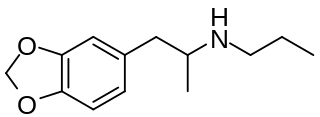
W3,4-Methylenedioxy-N-propylamphetamine is a lesser-known psychedelic drug and a substituted amphetamine. MDPR was first synthesized by Alexander Shulgin. In his book PiHKAL, the minimum dosage is listed as 200 mg, and the duration unknown. MDPR is a promoter; by itself it has almost no effects on the mind, but it promotes the effects of hallucinogens, similarly to the closely related MDPH.
 W
WMethylisopropyllysergamide is an analogue of LSD that was originally discovered by Albert Hofmann at Sandoz during the original structure-activity research into LSD. It has subsequently been investigated in more detail by the team led by David E. Nichols at Purdue University. Methylisopropyllysergamide is a structural isomer of LSD, with the alkyl groups on the amide nitrogen having been subjected to a methylene shuffle. MIPLA and its ethylisopropyl homologue are the only simple N,N-dialkyl lysergamides that approach the potency of LSD itself, being around 1/3-1/2 the potency of LSD, while all other dialkyl analogues tested are only around 1/10 as potent as LSD, although some N-monoalkyl lysergamides such as the sec-butyl and t-butyl derivatives were also found to show an activity profile and potency comparable to LSD, and the mono-isopropyl derivative is only slightly weaker than MIPLA. Apart from its lower potency, the hallucinogenic effects of methylisopropyllysergamide are similar to those of LSD itself, and the main use for this drug has been in studies of the binding site at the 5-HT2A receptor through which LSD exerts most of its pharmacological effects.
 W
WPARGY-LAD is an analogue of LSD. It is described by Alexander Shulgin in the book TiHKAL. PARGY-LAD is a hallucinogenic drug similar to LSD, but is considerably less potent than LSD with a dose of 160 micrograms producing only mild effects, and 500 micrograms required for full activity.
 W
WPRO-LAD is an analogue of LSD. It is described by Alexander Shulgin in the book TiHKAL. PRO-LAD is a psychedelic drug similar to LSD, and is around as potent as LSD itself with an active dose reported at between 100 and 200 micrograms.
 W
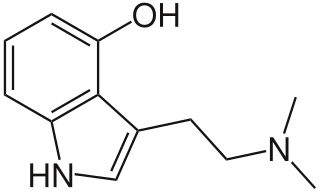
WPsilocin is a substituted tryptamine alkaloid and a serotonergic psychedelic substance. It is present in most psychedelic mushrooms together with its phosphorylated counterpart psilocybin. Psilocin is a Schedule I drug under the Convention on Psychotropic Substances. The mind-altering effects of psilocin are highly variable, subjective and resemble those of LSD and DMT.
 W
WPsilocybin is a naturally occurring psychedelic prodrug compound produced by more than 200 species of fungi. The most potent are members of the genus Psilocybe, such as P. azurescens, P. semilanceata, and P. cyanescens, but psilocybin has also been isolated from about a dozen other genera. As a prodrug, psilocybin is quickly converted by the body to psilocin, which has mind-altering effects similar, in some aspects, to those of LSD, mescaline, and DMT. In general, the effects include euphoria, visual and mental hallucinations, changes in perception, a distorted sense of time, and perceived spiritual experiences. It can also include possible adverse reactions such as nausea and panic attacks.
 W
WPsychedelia is an American documentary film from Hard Rain Films, that was initially released in 2015, and then re-released as a revised version in 2020. The film discusses the history of psychedelic drugs and their ability to produce mystical experiences. The therapeutic role of such drugs is considered, and controlled research studies conducted before the 1960s, are discussed. Several study participant users are interviewed. The film was a winner for best documentary film at the New Jersey International Film Festival, and was an official selection at the Southern Utah International Documentary Film Festival (DOCUTAH) and the Orlando Film Festival.
 W
WSalvia divinorum is a plant species with transient psychoactive properties when its leaves are consumed by chewing, smoking, or as a tea. The leaves contain opioid-like compounds that induce hallucinations. Because the plant has not been well-studied in high-quality clinical research, little is known about its toxicology, adverse effects, or safety over long-term consumption. Its native habitat is cloud forest in the isolated Sierra Mazateca of Oaxaca, Mexico, where it grows in shady, moist locations. The plant grows to over a meter high, has hollow square stems like others in the mint family Lamiaceae, large leaves, and occasional white flowers with violet calyxes. Botanists have not determined whether Salvia divinorum is a cultigen or a hybrid because native plants reproduce vegetatively and rarely produce viable seed.