 W
WThe acenes or polyacenes are a class of organic compounds and polycyclic aromatic hydrocarbons made up of linearly fused benzene rings. The larger representatives have potential interest in optoelectronic applications and are actively researched in chemistry and electrical engineering. Pentacene has been incorporated into organic field-effect transistors, reaching charge carrier mobilities as high as 5 cm2/Vs.
 W
WAnthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation.
 W
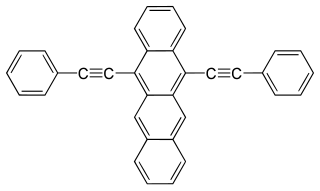
W9,10-Bis(phenylethynyl)anthracene (BPEA) is an aromatic hydrocarbon with the chemical formula is C30H18. It displays strong fluorescence and is used as a chemiluminescent fluorophore with high quantum efficiency.
 W
W5,12-Bis(phenylethynyl)naphthacene is a fluorescent dye used in lightsticks. It yields orange light.
 W
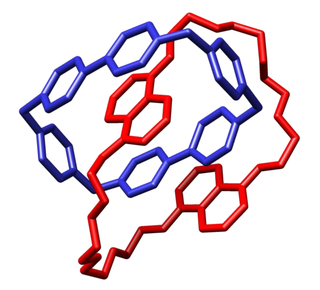
WA catenane is a mechanically-interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. Catenane is derived from the Latin catena meaning "chain". They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology "mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis.
 W
W1-Chloro-9,10-bis(phenylethynyl)anthracene is a fluorescent dye used in lightsticks. It emits yellow-green light, used in 30-minute high-intensity Cyalume sticks.
 W
W2-Chloro-9,10-bis(phenylethynyl)anthracene is a fluorescent dye used in lightsticks. It emits green light, used in 12-hour low-intensity Cyalume sticks.
 W
WConductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by dispersion. Conductive polymers are generally not thermoplastics, i.e., they are not thermoformable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques.
 W
WDiindenoperylene (DIP) is an organic semiconductor which receives attention because of its potential application in optoelectronics (solar cells, OLEDs) and electronics (RFID tags). DIP is a planar perylene derivative with two indeno-groups attached to opposite sides of the perylene core. Its chemical formula is C32H16, the full chemical name is diindeno[1,2,3-cd:1',2',3'-lm]perylene. Its chemical synthesis has been described.
 W
W9,10-Diphenylanthracene is a polycyclic aromatic hydrocarbon. It has the appearance of a slightly yellow powder. 9,10-Diphenylanthracene is used as a sensitiser in chemiluminescence. In lightsticks it is used to produce blue light. It is a molecular organic semiconductor, used in blue OLEDs and OLED-based displays.
 W
WAlan Jay Heeger is an American physicist, academic and Nobel Prize laureate in chemistry.
 W
WHexacene is an aromatic compound consisting of six linearly-fused benzene rings. It is a blue-green, air-stable solid with low solubility.
 W
WLutetium phthalocyanine is a coordination compound derived from lutetium and two phthalocyanines. It was the first known example of a molecule that is an intrinsic semiconductor. It exhibits electrochromism, changing color when subject to a voltage.
 W
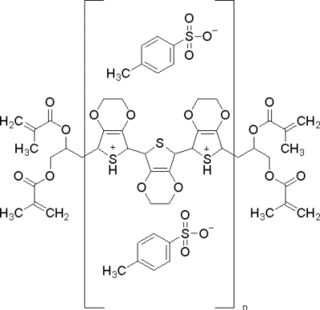
WPoly(3,4-ethylenedioxythiophene)-tetramethacrylate or PEDOT-TMA is a p-type conducting polymer based on 3,4-ethylenedioxylthiophene or the EDOT monomer. It is a modification of the PEDOT structure. Advantages of this polymer relative to PEDOT are that it is dispersible in organic solvents, and it is non-corrosive. PEDOT-TMA was developed under a contract with the National Science Foundation, and it was first announced publicly on April 12, 2004. The trade name for PEDOT-TMA is Oligotron. PEDOT-TMA was featured in an article entitled "Next Stretch for Plastic Electronics" that appeared in Scientific American in 2004. The U.S. Patent office issued a patent protecting PEDOT-TMA on April 22, 2008.
 W
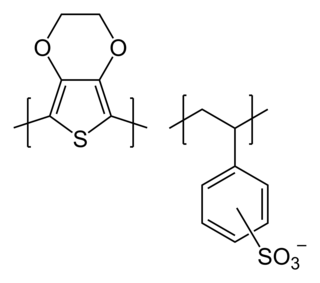
Wpoly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) is a polymer mixture of two ionomers. One component in this mixture is made up of sodium polystyrene sulfonate which is a sulfonated polystyrene. Part of the sulfonyl groups are deprotonated and carry a negative charge. The other component poly(3,4-ethylenedioxythiophene) (PEDOT) is a conjugated polymer and carries positive charges and is based on polythiophene. Together the charged macromolecules form a macromolecular salt.
 W
WPentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.
 W
WPerfluoropentacene (PFP) is an n-type organic semiconductor, which is made by fluorination of the p-type semiconductor pentacene. It has a blueish-black color, and is used for molecular thin-film devices.
 W
WPerylene or perilene is a polycyclic aromatic hydrocarbon with the chemical formula C20H12, occurring as a brown solid. It or its derivatives may be carcinogenic, and it is considered to be a hazardous pollutant. In cell membrane cytochemistry, perylene is used as a fluorescent lipid probe. It is the parent compound of a class of rylene dyes.
 W
WPoly(3,4-ethylenedioxythiophene) is a conducting polymer based on 3,4-ethylenedioxythiophene or EDOT. It was first reported by Bayer AG in 1989.
 W
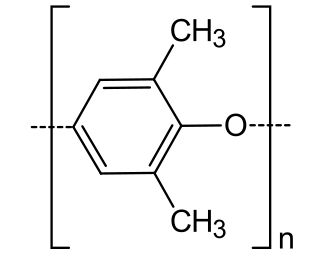
WPoly(p-phenylene oxide) or poly(p-phenylene ether) (PPE) is a high-temperature thermoplastic. It is rarely used in its pure form due to difficulties in processing. It is mainly used as blend with polystyrene, high impact styrene-butadiene copolymer or polyamide. PPO is a registered trademark of SABIC Innovative Plastics IP B.V. under which various polyphenylene ether resins are sold.
 W
WPolyphenylene sulfide (PPS) is an organic polymer consisting of aromatic rings linked by sulfides. Synthetic fiber and textiles derived from this polymer resist chemical and thermal attack. PPS is used in filter fabric for coal boilers, papermaking felts, electrical insulation, film capacitors, specialty membranes, gaskets, and packings. PPS is the precursor to a conductive polymer of the semi-flexible rod polymer family. The PPS, which is otherwise insulating, can be converted to the semiconducting form by oxidation or use of dopants.
 W
WPoly(p-phenylene vinylene) is a conducting polymer of the rigid-rod polymer family. PPV is the only polymer of this type that can be processed into a highly ordered crystalline thin film. PPV and its derivatives are electrically conducting upon doping. Although insoluble in water, its precursors can be manipulated in aqueous solution. The small optical band gap and its bright yellow fluorescence makes PPV a candidate in applications such as light-emitting diodes (LED) and photovoltaic devices. Moreover, PPV can be doped to form electrically conductive materials. Its physical and electronic properties can be altered by the inclusion of functional side groups.
 W
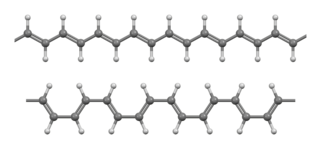
WPolyacetylene (IUPAC name: polyethyne) usually refers to an organic polymer with the repeating unit (C2H2)n. The name refers to its conceptual construction from polymerization of acetylene to give a chain with repeating olefin groups. This compound is conceptually important, as the discovery of polyacetylene and its high conductivity upon doping helped to launch the field of organic conductive polymers. The high electrical conductivity discovered by Hideki Shirakawa, Alan Heeger, and Alan MacDiarmid for this polymer led to intense interest in the use of organic compounds in microelectronics (organic semiconductors). This discovery was recognized by the Nobel Prize in Chemistry in 2000. Early work in the field of polyacetylene research was aimed at using doped polymers as easily processable and lightweight "plastic metals". Despite the promise of this polymer in the field of conductive polymers, many of its properties such as instability to air and difficulty with processing have led to avoidance in commercial applications.
 W
WPolyaniline (PANI) is a conducting polymer and organic semiconductor of the semi-flexible rod polymer family. The compound has been of interest since the 1980s because of its electrical conductivity and mechanical properties. Polyaniline is one of the most studied conducting polymers.
 W
WPolypyrrole (PPy) is an organic polymer obtained by oxidative polymerization of pyrrole. It is a solid with the formula H(C4H2NH)nH. It is an intrinsically conducting polymer, used in electronics, optical, biological and medical fields.
 W
WPolysilanes are organosilicon compounds with the formula (R2Si)n. They are relatives of traditional organic polymers but their backbones are composed of silicon atoms. They exhibit distinctive optical and electrical properties. They are mainly used industrially as precursors to silicon carbide. The simplest polysilane, (SiH2)n, is known as polysilene.
 W
WPolythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.
 W
WQuinacridone is an organic compound used as a pigment. Numerous derivatives constitute the quinacridone pigment family, which finds extensive use in industrial colorant applications such as robust outdoor paints, inkjet printer ink, tattoo inks, artists' watercolor paints, and color laser printer toner. As pigments, the quinacridones are insoluble. The development of this family of pigments supplanted the alizarin dyes.
 W
WA rotaxane is a mechanically interlocked molecular architecture consisting of a "dumbbell shaped molecule" which is threaded through a "macrocycle". The name is derived from the Latin for wheel (rota) and axle (axis). The two components of a rotaxane are kinetically trapped since the ends of the dumbbell are larger than the internal diameter of the ring and prevent dissociation (unthreading) of the components since this would require significant distortion of the covalent bonds.
 W
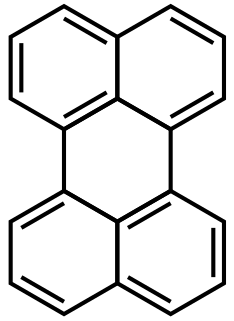
WRubrene (5,6,11,12-tetraphenyltetracene) is a red colored polycyclic aromatic hydrocarbon. Rubrene is used as a sensitiser in chemoluminescence and as a yellow light source in lightsticks.
 W
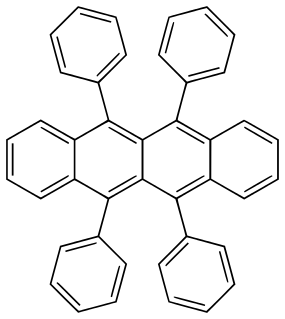
WTetracene, also called naphthacene, is a polycyclic aromatic hydrocarbon. It has the appearance of a pale orange powder. Tetracene is the four-ringed member of the series of acenes. Tetracene is a molecular organic semiconductor, used in organic field-effect transistors (OFETs) and organic light-emitting diodes (OLEDs). In May 2007, researchers from two Japanese universities, Tohoku University in Sendai and Osaka University, reported an ambipolar light-emitting transistor made of a single tetracene crystal. Ambipolar means that the electric charge is transported by both positively charged holes and negatively charged electrons. Tetracene can be also used as a gain medium in dye lasers as a sensitiser in chemoluminescence.
 W
WTetrathiafulvalene is an organosulfur compound with the formula (H2C2S2C)2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, (C5H4)2, by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives.