 W
WThe United States Food and Drug Administration is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed and veterinary products.
 W
WAn Abbreviated New Drug Application (ANDA) is an application for a U.S. generic drug approval for an existing licensed medication or approved drug.
 W
WThe Animal Drug and Animal Generic Drug User Fee Reauthorization Act of 2013 is a bill that was introduced into the United States Senate during the 113th United States Congress. The bill would authorize the collection of fees by the Food and Drug Administration for use to fund activities related to the approval of drugs for animals. The bill would amend the Federal Food, Drug, and Cosmetic Act.
 W
WBiomaterials Access Assurance Act of 1998 is a United States federal statute establishing liability exemptions for biomaterial suppliers selling chemical components and raw materials utilized in implantable devices for human recipients. The United States federal legislation sets forth rules limiting litigation costs or unwarranted lawsuits for biomaterial suppliers excluded from the design, production, and testing of implantable devices demonstrated as effective and safe to include adequate product warnings.
 W
WThe Center for Drug Evaluation and Research is a division of the U.S. Food and Drug Administration (FDA) that monitors most drugs as defined in the Food, Drug, and Cosmetic Act. Some biological products are also legally considered drugs, but they are covered by the Center for Biologics Evaluation and Research. The center reviews applications for brand name, generic, and over the counter pharmaceuticals, manages US current Good Manufacturing Practice (cGMP) regulations for pharmaceutical manufacturing, determines which medications require a medical prescription, monitors advertising of approved medications, and collects and analyzes safety data about pharmaceuticals that are already on the market.
 W
WThe Center for Veterinary Medicine (CVM) is a branch of the U.S. Food and Drug Administration (FDA) that regulates the manufacture and distribution of food, food additives, and drugs that will be given to animals. These include animals from which human foods are derived, as well as food additives and drugs for pets or companion animals. CVM is responsible for regulating drugs, devices, and food additives given to, or used on, over one hundred million companion animals, plus millions of poultry, cattle, swine, and minor animal species. Minor animal species include animals other than cattle, swine, chickens, turkeys, horses, dogs, and cats.
 W
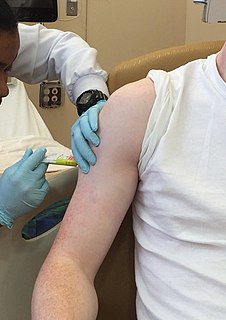
WClinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.
 W
WThe Dietary Supplement Health and Education Act of 1994 ("DSHEA"), is a 1994 statute of United States Federal legislation which defines and regulates dietary supplements. Under the act, supplements are effectively regulated by the FDA for Good Manufacturing Practices under 21 CFR Part 111.
 W
WA drug coupon is a coupon intended to help consumers save money on pharmaceutical drugs. They are offered by drug companies or distributed to consumers via doctors and pharmacists, and most can be obtained online. There are drug coupons for drugs from many categories such as cholesterol, acne, migraine, allergies, etc.
 W
WElixir sulfanilamide was an improperly prepared sulfonamide antibiotic that caused mass poisoning in the United States in 1937. It caused the deaths of more than 100 people. The public outcry caused by this incident and other similar disasters led to the passing of the 1938 Federal Food, Drug, and Cosmetic Act, which significantly increased the Food and Drug Administration's powers to regulate drugs.
 W
WFast track is a designation by the United States Food and Drug Administration (FDA) of an investigational drug for expedited review to facilitate development of drugs which treat a serious or life-threatening condition and fill an unmet medical need. Fast Track designation must be requested by the drug company. The request can be initiated at any time during the drug development process. FDA will review the request and attempt to make a decision within sixty days.
 W
WThe Center for Devices and Radiological Health (CDRH) is the branch of the United States Food and Drug Administration (FDA) responsible for the premarket approval of all medical devices, as well as overseeing the manufacturing, performance and safety of these devices. The CDRH also oversees the radiation safety performance of non-medical devices which emit certain types of electromagnetic radiation, such as cellular phones and microwave ovens.
 W
WFDA Consumer was a magazine published from 1967 through 2007 by the U.S. Food and Drug Administration (FDA). From 1967 to 1972 it was known as FDA Papers before changing title to FDA Consumer with volume 6 no. 6.
 W
WThe Food Safety Modernization Act (FSMA) was signed into law by President Barack Obama on January 4, 2011. The FSMA has given the Food and Drug Administration (FDA) new authority to regulate the way foods are grown, harvested and processed. The law grants the FDA a number of new powers, including mandatory recall authority, which the agency has sought for many years. The FSMA requires the FDA to undertake more than a dozen rulemakings and issue at least 10 guidance documents, as well as a host of reports, plans, strategies, standards, notices, and other tasks.
 W
WThe United States Federal Food, Drug, and Cosmetic Act is a set of laws passed by Congress in 1938 giving authority to the U.S. Food and Drug Administration (FDA) to oversee the safety of food, drugs, medical devices, and cosmetics. A principal author of this law was Royal S. Copeland, a three-term U.S. senator from New York. In 1968, the Electronic Product Radiation Control provisions were added to the FD&C. Also in that year the FDA formed the Drug Efficacy Study Implementation (DESI) to incorporate into FD&C regulations the recommendations from a National Academy of Sciences investigation of effectiveness of previously marketed drugs. The act has been amended many times, most recently to add requirements about bioterrorism preparations.
 W
WThe Food Additives Amendment of 1958 is a 1958 amendment to the United States' Food, Drugs, and Cosmetic Act of 1938. It was a response to concerns about the safety of new food additives. The amendment established an exemption from the "food additive" definition and requirements for substances "generally recognized as safe" by scientific experts in the field, based on long history of use before 1958 or based on scientific studies. New food additives would be subject to testing including by the "Delaney clause". The Delaney clause was a provision in the amendment which said that if a substance were found to cause cancer in man or animal, then it could not be used as a food additive.
 W
WPresident of the United States George W. Bush signed the Food and Drug Administration Amendments Act of 2007 (FDAAA) on September 27, 2007. This law reviewed, expanded, and reaffirmed several existing pieces of legislation regulating the FDA. These changes allow the FDA to perform more comprehensive reviews of potential new drugs and devices. It was sponsored by Reps. Joe Barton and Frank Pallone and passed unanimously by the Senate.
 W
WThe United States Food and Drug Administration Modernization Act of 1997 (FDAMA) amended the Federal Food, Drug, and Cosmetic Act. This act is related to the regulation of food, drugs, devices, and biological products by the FDA. These changes were made in order to recognize the changes in the way the FDA would be operating in the 21st century. The main focus of this is the acknowledgment in the advancement of technological, trade, and public health complexities.
 W
WThe Food and Drug Administration Revitalization Act was introduced by the 101st Congress of the United States. Senator Orrin G. Hatch was the chairperson sponsor of the federal revitalization amendment for the Food and Drug Administration.
 W
WThe Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA) is a piece of American regulatory legislation signed into law on July 9, 2012. It gives the United States Food and Drug Administration (FDA) the authority to collect user fees from the medical industry to fund reviews of innovator drugs, medical devices, generic drugs and biosimilar biologics. It also creates the breakthrough therapy designation program and extends the priority review voucher program to make eligible rare pediatric diseases. The measure was passed by 96 senators voting for and one voting against.
 W
WGenerally recognized as safe (GRAS) is a United States Food and Drug Administration (FDA) designation that a chemical or substance added to food is considered safe by experts. An ingredient with a GRAS designation is exempted from the usual Federal Food, Drug, and Cosmetic Act (FFDCA) food additive tolerance requirements. The concept of food additives being "generally recognized as safe" was first described in the Food Additives Amendment of 1958, and all additives introduced after this time had to be evaluated by new standards. The FDA list of GRAS notices is updated approximately each month, as of 2021.
 W
WThe United States Food and Drug Administration's Investigational New Drug (IND) program is the means by which a pharmaceutical company obtains permission to start human clinical trials and to ship an experimental drug across state lines before a marketing application for the drug has been approved. Regulations are primarily at 21 CFR 312. Similar procedures are followed in the European Union, Japan, and Canada.
 W
WThe Medical Device Regulation Act or Medical Device Amendments of 1976 was introduced by the 94th Congress of the United States. Congressman Paul G. Rogers and Senator Edward M. Kennedy were the chairperson sponsors of the medical device amendments. The Title 21 amendments were signed into law on May 28, 1976, by the 38th President of the United States Gerald R. Ford.
 W
WMedWatch is the Food and Drug Administration’s “Safety Information and Adverse Event Reporting Program.” It interacts with the FDA Adverse Event Reporting System. MedWatch is used for reporting an adverse event or sentinel event. Founded in 1993, this system of voluntary reporting allows such information to be shared with the medical community or the general public. The system includes publicly available databases and online analysis tools for professionals. MedWatch also distributes information on medical recalls and other clinical safety communications via its platforms.
 W
WThe National Center for Toxicological Research (NCTR) is the branch of the United States Food and Drug Administration which conducts research to define biological mechanisms of action underlying the toxicity of products regulated by the FDA. Its director is William Slikker, Jr., PhD.
 W
WThe Food and Drug Administration (FDA)'s New Drug Application (NDA) is the vehicle in the United States through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing. Some 30% or less of initial drug candidates proceed through the entire multi-year process of drug development, concluding with an approved NDA, if successful.
 W
WThe Office of Global Regulatory Operations and Policy (GO), also known as the Office of Regulatory Affairs (ORA), is the part of the U.S. Food and Drug Administration (FDA) enforcing the federal laws governing biologics, cosmetics, dietary supplements, drugs, food, medical devices, radiation-emitting electronic devices, tobacco products, and veterinary medicine products which may have potentially harmful side effects for the consumer.
 W
WAn orally disintegrating tablet or orally dissolving tablet (ODT) is a drug dosage form available for a limited range of over-the-counter (OTC) and prescription medications. ODTs differ from traditional tablets in that they are designed to be dissolved on the tongue rather than swallowed whole. The ODT serves as an alternative dosage form for patients who experience dysphagia or for where compliance is a known issue and therefore an easier dosage form to take ensures that medication is taken. Common among all age groups, dysphagia is observed in about 35% of the general population, as well as up to 60% of the elderly institutionalized population and 18-22% of all patients in long-term care facilities ODTs may have a faster onset of effect than tablets or capsules, and have the convenience of a tablet that can be taken without water. During the last decade, ODTs have become available in a variety of therapeutic markets, both OTC and by prescription.
 W
WThe Orphan Drug Act of 1983 is a law passed in the United States to facilitate development of orphan drugs—drugs for rare diseases such as Huntington's disease, myoclonus, ALS, Tourette syndrome and muscular dystrophy which affect small numbers of individuals residing in the United States.
 W
WThe Over-the-Counter Hearing Aid Act of 2017 was a law passed by the 115th United States Congress as a rider on the FDA Reauthorization Act of 2017. It created a class of hearing aids regulated by the Food and Drug Administration (FDA) available directly to consumers without involvement from a licensed professional. Regulations for this new class of hearing aid are expected to be released by the end of 2020.
 W
WThe Prescription Drug User Fee Act (PDUFA) was a law passed by the United States Congress in 1992 which allowed the Food and Drug Administration (FDA) to collect fees from drug manufacturers to fund the new drug approval process. The Act provided that the FDA was entitled to collect a substantial application fee from drug manufacturers at the time a New Drug Application (NDA) or Biologics License Application (BLA) was submitted, with those funds designated for use only in Center for Drug Evaluation and Research (CDER) or Center for Biologics Evaluation and Research (CBER) drug approval activities. In order to continue collecting such fees, the FDA is required to meet certain performance benchmarks, primarily related to the speed of certain activities within the NDA review process.
 W
WThe Pure Food and Drug Act of 1906 was the first of a series of significant consumer protection laws which was enacted by Congress in the 20th century and led to the creation of the Food and Drug Administration. Its main purpose was to ban foreign and interstate traffic in adulterated or mislabeled food and drug products, and it directed the U.S. Bureau of Chemistry to inspect products and refer offenders to prosecutors. It required that active ingredients be placed on the label of a drug's packaging and that drugs could not fall below purity levels established by the United States Pharmacopeia or the National Formulary.
 W
WRactopamine is an animal feed additive used to promote leanness and increase food conversion efficiency in farmed animals in some countries, but banned in others. Pharmacologically, it is a phenol-based TAAR1 agonist and β adrenoreceptor agonist that stimulates β1 and β2 adrenergic receptors. It is most commonly administered to animals for meat production as ractopamine hydrochloride. It is the active ingredient in products marketed in the US as Paylean for swine, Optaflexx for cattle, and Topmax for turkeys. It was developed by Elanco Animal Health, a division of Eli Lilly and Company.
 W
WRadiation Control for Health and Safety Act of 1968 was an amendment to the Public Health Service Act mandating performance standards for electronic products suspectible of electromagnetic radiation or radiation emissions. The United States statute established provisions involving research and development programs for the studies of electromagnetic shielding, ionizing radiation, non-ionizing radiation, and exposure assessment to humans.
 W
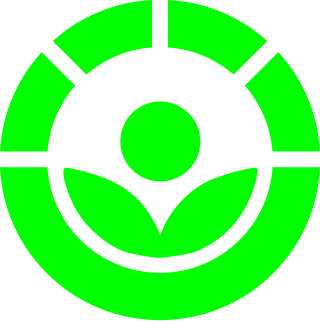
WThe Radura is the international symbol indicating a food product has been irradiated. The Radura is usually green and resembles a plant in circle. The top half of the circle is dashed. Graphical details and colours vary between countries.
 W
WRegulation of tobacco by the U.S. Food and Drug Administration began in 2009 with the passage of the Family Smoking Prevention and Tobacco Control Act by the United States Congress. With this statute, the Food and Drug Administration (FDA) was given the ability to regulate tobacco products.
 W
WSafe Medical Device Amendments of 1990 or Safe Medical Devices Act sanctioned progressive reporting and tracking rules for medical devices classified by the Medical Device Regulation Act. The Act mandates reporting requirements by medical device manufacturers regarding adverse safety events and product effectiveness of devices classified as substantially equivalent to Class III medical devices. The United States Statute established the Health and Human Services Office of International Relations and a U.S. Food and Drug Administration office for regulatory activities concerning healthcare products which are considered a combinational biological, device, or drug product. The Act of Congress transferred the electronic product radiation control provisions established by the Radiation Control for Health and Safety Act.
 W
WThe Safeguarding Therapeutics Act is a U.S. federal law that was passed by Congress in 2020 that allows the government to destroy counterfeit medical devices.
 W
WTea Importation Act of 1897 was a United States public law forbidding the import of tea into the United States with excessive levels of fluoride, heavy metals, oxalate, and pesticides. The Act of Congress established a uniform standard of purity and quality while attempting to achieve the optimal health effects of tea and phenolic content in tea. The statute declared it unlawful to import into the United States "any merchandise as tea which is inferior in purity, quality, and fitness for consumption to the standards kept at customhouses..." For nearly a century, Congress provided that no imported tea could enter the United States unless federal tea-tasters decided that it measured up to preselected standard samples. The law restricted the International trade of camellia sinensis.